An overactive inflammatory response could be at the root of many long COVID cases, according to a new study from the Allen Institute and Fred Hutchinson Cancer Center.
Molecular footprint of the chronic condition could help guide clinical trial and treatment decisions
SEATTLE, June 12, 2023 /PRNewswire/ -- An overactive inflammatory response could be at the root of many long COVID cases, according to a new study from the Allen Institute and Fred Hutchinson Cancer Center.
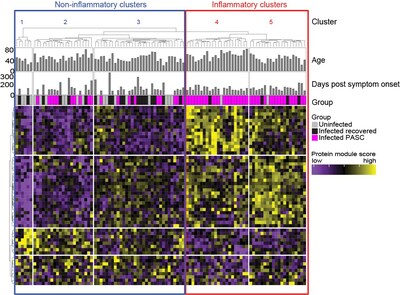
Looking at proteins circulating in the blood, the scientists found a set of molecules associated with inflammation that were present only in a subset of patients with long COVID and were not seen in those who recovered from their disease. The researchers published an article describing their findings in the journal Nature Communications today.
Out of 55 patients with long COVID, about two-thirds had persistently high levels of certain signals of inflammation. The scientists also looked at blood samples from 25 people who had COVID but recovered, and from 25 volunteers who had never had COVID to their knowledge. Those without long COVID did not show the same signs of inflammation in their blood.
The patient volunteers in the new analysis are part of a larger, ongoing study based at Fred Hutch, the Seattle COVID Cohort Study, which is led by Julie McElrath, M.D., Ph.D., Senior Vice President and Director of Fred Hutch's Vaccine and Infectious Disease Division, and Julie Czartoski, ARNP, Research Clinician at the Hutch.
Scientists have seen previous links between inflammation and long COVID, but the new study is the first to trace the persistence of these inflammatory markers over time in the same patients.
There's an obvious implication to these findings, said Troy Torgerson, M.D., Ph.D., Director of Experimental Immunology at the Allen Institute for Immunology, a division of the Allen Institute: Certain kinds of anti-inflammatory drugs might alleviate symptoms for some long COVID patients. But physicians need a way of telling which long COVID patients might benefit from which treatment — a form of precision medicine for a disease that so far remains maddeningly mysterious.
"The big question was, can we define which long COVID patients have persistent inflammation versus those that don't? That would be useful in terms of clinical trial planning and in terms of helping clinicians figure out targeted treatments for their patients," said Torgerson, who led the Nature Communications publication along with McElrath, Aarthi Talla, Senior Bioinformatician at the Allen Institute for Immunology, Suhas Vasaikar, Ph.D., former Senior Bioinformatics Scientist (now a Principal Scientist at Seagen), and Tom Bumol, Ph.D., former Executive Vice President and Director.
Specifically, the blood markers uncovered in this subset of patients with "inflammatory long COVID," as the scientists call it, point to a flavor of inflammation similar to that seen in autoimmune diseases like rheumatoid arthritis. This kind of inflammation can be treated with an existing class of drugs called JAK inhibitors, at least in the case of rheumatoid arthritis (it has not yet been tested for long COVID).
The scientists also hope to narrow down their molecular signature of "inflammatory long COVID" to a few markers that could be used in the clinic to sort this subset of long COVID patients out from the rest.
Refining treatment options
Launched in the spring of 2020, shortly after the COVID-19 pandemic shut down businesses and schools in the U.S., the Fred Hutch-led Seattle COVID Cohort Study was originally designed to follow immune responses over time in patients with mild or moderate COVID. The idea was to capture details of a "successful" immune response — one in which patients didn't get too sick, didn't need to be hospitalized, and recovered fully.
But the team soon realized that even among those who didn't get super sick, not everyone recovered. In their initial work in 2020 tracing the details of immune responses in 18 COVID patients, the scientists found a handful whose symptoms persisted, early examples of what would eventually be termed long-haul COVID, or just long COVID.
In those early days of the study, the scientists saw that certain immune responses — namely inflammation — were consistently high in these few patients with long COVID. In the patients that got sick and then recovered fully, inflammation levels went up as their bodies fought off the illness, and then went back down as they got better. In those with long COVID, the levels never went back down.
So the team decided to expand their study to look at more patients with long COVID, focusing on a panel of 1500 proteins circulating in the blood. These assays revealed different molecular "buckets" of long COVID, namely inflammatory and non-inflammatory long COVID. Understanding the molecular roots of the disease, or subsets of the disease, will help guide clinical trial design and ultimately treatment decisions, the scientists said.
"The ultimate goal is to treat patients," Talla said. "Although we call everything long COVID, what's come out of this work shows us that we might not be able to give everyone the same kinds of therapies and we shouldn't put everyone into one group for treatment purposes."
Those patients with non-inflammatory long COVID might be living with permanent organ or tissue damage from their disease, Torgerson said. That would require very different treatment from those with high levels of inflammation. The scientists also saw that these groups can't be distinguished based on symptoms alone. If anti-inflammatory drugs prove effective in treating inflammatory long COVID, patients would first need to be screened to determine which form of long COVID they have.
"We hope these findings provide features of long COVID that may guide potential future therapeutic approaches," McElrath said.
About the Allen Institute
The Allen Institute is an independent, 501(c)(3) nonprofit research organization founded by philanthropist and visionary, the late Paul G. Allen. The Allen Institute is dedicated to answering some of the biggest questions in bioscience and accelerating research worldwide. The Institute is a recognized leader in large-scale research with a commitment to an open science model. Its research institutes and programs include the Allen Institute for Brain Science, launched in 2003, the Allen Institute for Cell Science, launched in 2014, the Allen Institute for Immunology, launched in 2018, the MindScope Program, launched in 2020, and the Allen Institute for Neural Dynamics, launched in 2021. In 2016, the Allen Institute expanded its reach with the launch of The Paul G. Allen Frontiers Group, which identifies pioneers with new ideas to expand the boundaries of knowledge and make the world better. For more information, visit alleninstitute.org.
Media Contact
Peter Kim, Sr. Manager, Media Relations
206.605.9884 | peter.kim@alleninstitute.org
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/many-long-covid-patients-suffer-from-persistent-inflammation-study-finds-301847606.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/many-long-covid-patients-suffer-from-persistent-inflammation-study-finds-301847606.html
SOURCE Allen Institute