Maryland
Looking for a research and development job? Check out the BioSpace list of 12 companies hiring life sciences professionals like you.
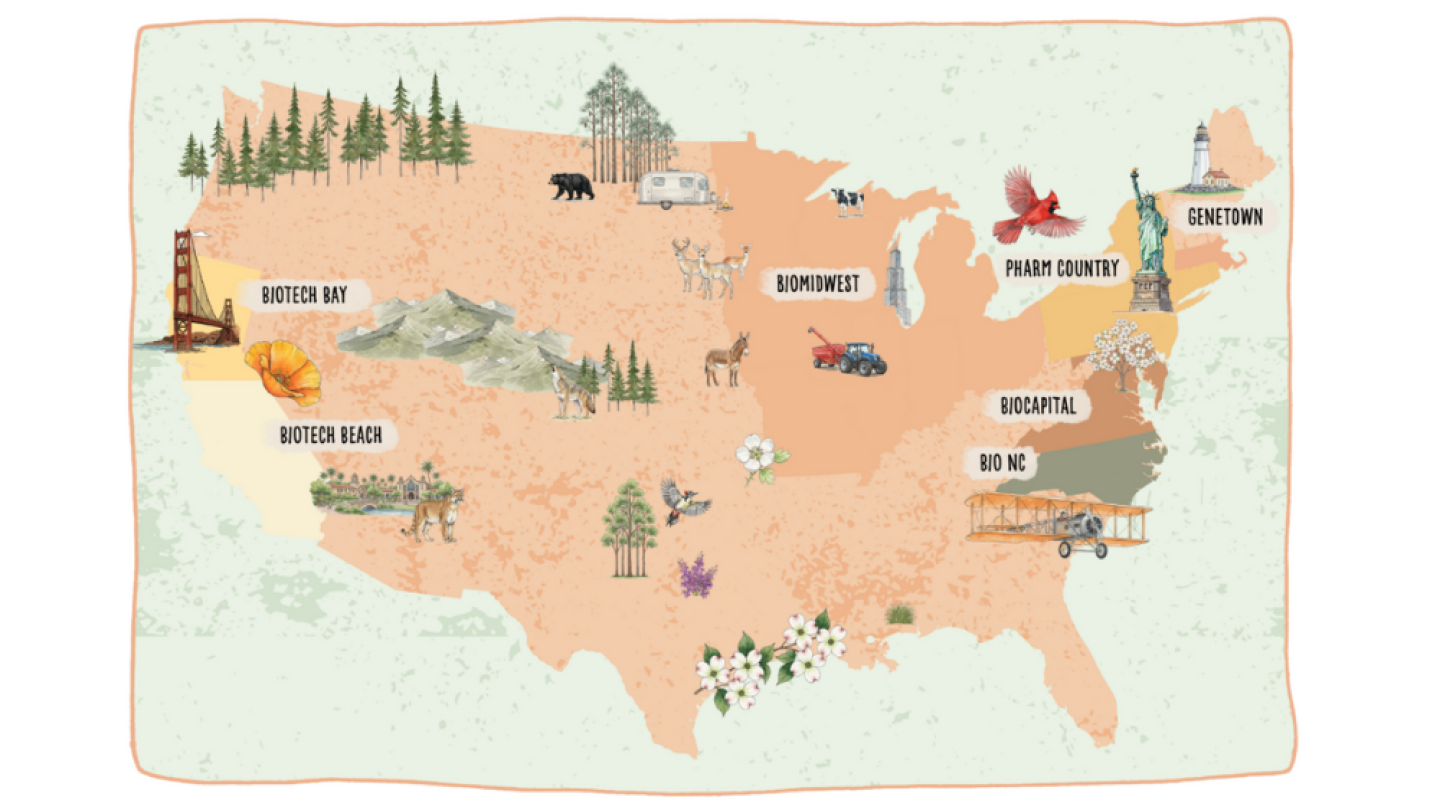
BioSpace has revealed its new 2026 Hotbed Maps, showcasing seven regional life sciences hot spots.
In 2025, made or projected biopharma workforce cuts affected about 42,700 employees, according to BioSpace tallies. BioSpace takes a deep dive into which companies and locations were impacted and speaks to experts about what to expect ahead—and why.
The Washington, D.C.–Baltimore area ranks in the top six for life sciences R&D and manufacturing talent, according to a CBRE report. The Maryland Department of Commerce’s director of life sciences discusses the workforce and how the state is adapting to changing needs.
During the first quarter, 22 rounds of biopharma layoffs in California affected about 995 employees total, while 17 rounds in Massachusetts impacted around 410 people, based on BioSpace estimates. Meanwhile, competition for jobs in those states increased year over year, according to BioSpace data.
BioSpace has revealed its 2025 Hotbed Maps, showcasing nine regional hot spots for life sciences activity.
At Johns Hopkins University, the biomedical engineering program’s Design Team offering lets undergraduates dive deep into clinical projects that can help them land industry jobs, get provisional patents or even start companies.
Multiple players are exploring whether modalities designed to combat B cell malignancies can be repurposed against lupus, myasthenia gravis and other conditions traced to misdirected immune response.
United States Pharmacopeia is recruiting expert volunteers from academia, industry, regulatory and healthcare to develop, revise and approve medicine, dietary supplement and food ingredient standards and solutions used in more than 150 countries to improve global public health. The volunteers will serve from 2025 to 2030.
Unlike Pfizer/BioNTech and Moderna, Novavax does not use mRNA technology for its COVID-19 vaccine, instead opting for a recombinant version of the virus’ spike protein to elicit protection.
PRESS RELEASES