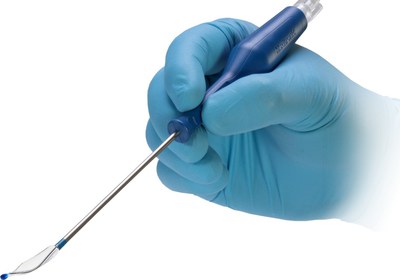
Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced the launch of the NuVent™ Eustachian tube dilation balloon, which has been cleared by the U.S. Food and Drug Administration (FDA) for the treatment of chronic, obstructive Eustachian Tube Dysfunction.
|
DUBLIN, Feb. 28, 2022 /PRNewswire/ -- Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced the launch of the NuVent™ Eustachian tube dilation balloon, which has been cleared by the U.S. Food and Drug Administration (FDA) for the treatment of chronic, obstructive Eustachian Tube Dysfunction. The NuVent™ balloon enables surgeons to deliver treatment in an outpatient or office setting. It features a flexible balloon section that allows customized placement based on patient anatomy. "Patients who suffer from Eustachian Tube Dysfunction often experience pain, pressure, and hearing difficulties, so it's important to address their illness quickly," said Dr. Boris Karanfilov, a rhinologist and head of the Ohio Sinus Institute in Dublin, Ohio. "Balloon dilation restores proper Eustachian tube function and reduces these symptoms, plus the ability to perform the procedure in the office makes it both convenient and efficient." It is estimated that 4.6% of adults in the United States experience Eustachian Tube Dysfunction.1 It occurs when the Eustachian tube, which links the back of the nose to the middle ear, fails to open or close properly. As a result, the tube is unable to perform its primary functions, which are protecting the middle ear from pathogens, equalizing air pressure on either side of the eardrum, and helping drain secretions from the middle ear cleft. This may result in pain, hearing difficulty, and/or a feeling of fullness in the ears. If not treated, patients may also suffer damage to the middle ear and eardrum.2 "Patients with persistent Eustachian Tube Dysfunction – especially those who experience frequent pressure changes, like airline travelers and divers – often require more than nasal sprays and oral medication to treat the condition," said Dr. Sina Joorabchi, an otolaryngologist at South Florida Ear, Nose, and Throat Associates. "The NuVent™ balloon provides an effective, minimally invasive treatment option that can be administered in the office." "As office-based procedures for ENT conditions increase, we look forward to introducing more innovative technologies designed specifically for this setting," said Vince Racano, president of the Ear, Nose, and Throat business, which is part of the Neuroscience Portfolio at Medtronic. "The NuVent™ balloon is another important product within this expanding portfolio." About Medtronic Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results. References
SOURCE Medtronic plc |
||||||||||||||||
Company Codes: NYSE:MDT |