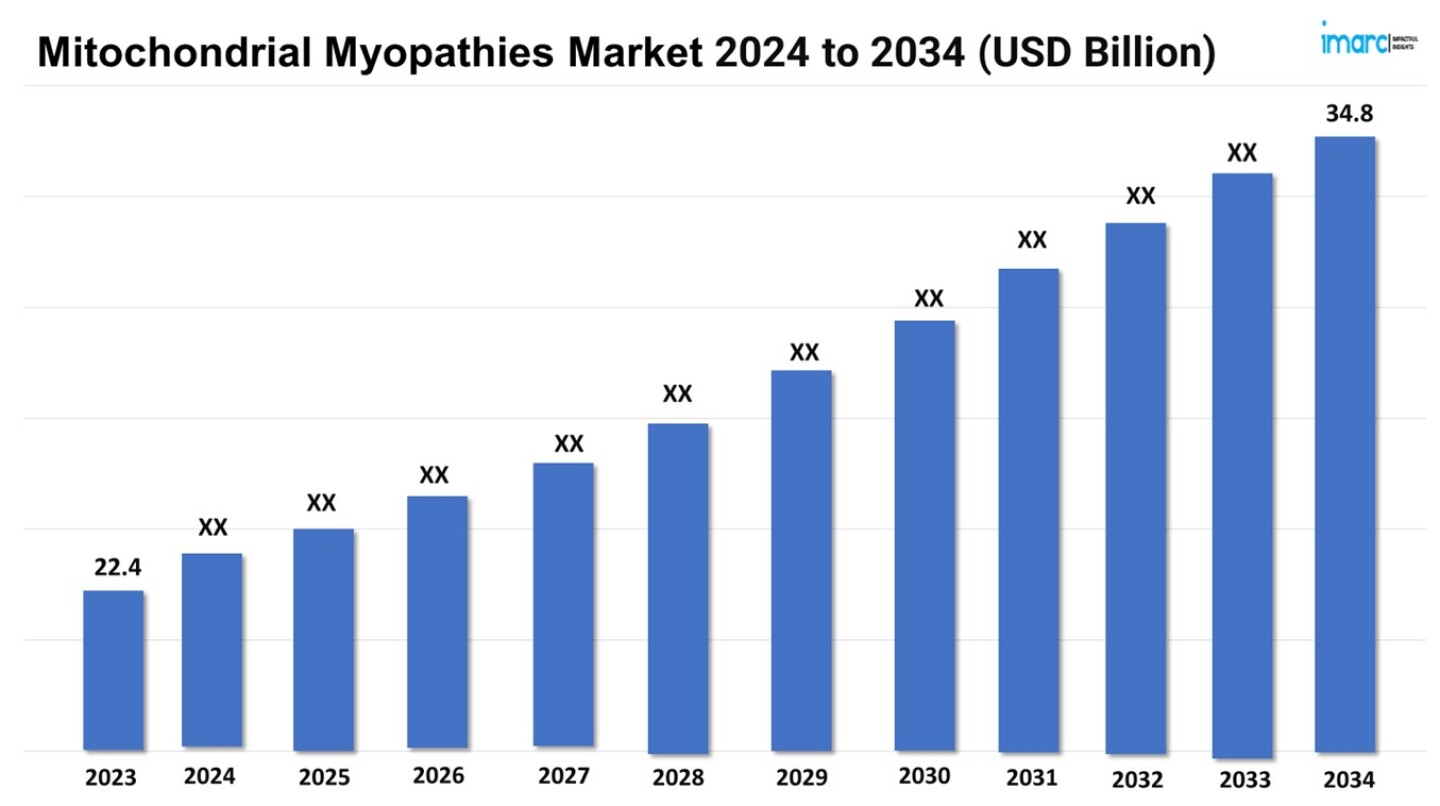
The mitochondrial myopathies market size reached a value of USD 22.4 Million in 2023. Looking forward, the market is expected reach USD 34.8 Million by 2034, exhibiting a growth rate (CAGR) of 4.07% during 2024-2034.
The market is driven by increased research and development efforts. Additionally, there is a growing focus on personalized medicine approaches, tailoring treatments to individual genetic profiles, which further propels the market expansion.
Advancements in Gene Therapy: Driving the Mitochondrial Myopathies Market
The mitochondrial myopathies market is witnessing remarkable progress, primarily due to advancements in gene therapy. Gene therapy aims to address the underlying genetic defects causing mitochondrial myopathies, offering hope for more effective and potentially curative treatments. One notable advancement is the development of mitochondrial gene replacement therapy, which involves delivering functional copies of defective genes to patient cells. For instance, the use of adeno-associated virus (AAV) vectors has shown promise in preclinical studies. These vectors can efficiently target and deliver therapeutic genes to mitochondria, improving cellular function. A significant example of this is the gene therapy approach being developed for Leigh syndrome, a severe mitochondrial disorder. Researchers have successfully used AAV vectors to deliver the ND4 gene to affected cells, resulting in improved mitochondrial function and extended survival in animal models.
Request a PDF Sample Report: https://www.imarcgroup.com/mitochondrial-myopathies-market/requestsample
Another critical advancement in gene therapy for mitochondrial myopathies is the use of mitochondrial-targeted RNA therapeutics. This approach involves designing RNA molecules that can selectively bind and correct mutations in mitochondrial DNA (mtDNA). For instance, mitochondrial-targeted RNA import systems have been developed to introduce therapeutic RNA into mitochondria, effectively restoring normal function. A groundbreaking instance is the research on using peptide nucleic acids (PNAs) conjugated with mitochondrial targeting sequences to correct specific point mutations in mtDNA. These PNAs have shown the ability to selectively bind and repair mutated mtDNA, leading to significant functional improvements in cellular and animal models. Additionally, the CRISPR/Cas9 gene-editing technology is being explored for its potential to precisely edit mtDNA mutations, offering a powerful tool for developing targeted therapies. These advancements in gene therapy, coupled with ongoing research and clinical trials, hold great promise for transforming the treatment landscape for mitochondrial myopathies, providing patients with more effective and long-lasting solutions.
Enhanced Diagnostic Techniques: Contributing to Market Expansion
The mitochondrial myopathies market is significantly benefiting from advancements in diagnostic techniques, enabling earlier and more accurate detection of these complex disorders. One of the most notable advancements is the use of next-generation sequencing (NGS) technologies. NGS allows for comprehensive analysis of the entire mitochondrial genome as well as nuclear genes associated with mitochondrial function. This high-throughput technology can identify rare and novel mutations with unprecedented speed and accuracy. For example, whole-exome sequencing (WES) and whole-genome sequencing (WGS) have become invaluable tools in diagnosing mitochondrial myopathies, allowing for the identification of mutations that were previously undetectable with traditional methods. This precise genetic information is crucial for guiding treatment decisions and genetic counseling for affected families.
In addition to NGS, other enhanced diagnostic techniques such as muscle biopsy with histochemical analysis and advanced imaging technologies are improving the accuracy of mitochondrial myopathy diagnoses. Muscle biopsies, when combined with immunohistochemistry and electron microscopy, provide detailed insights into the structural and functional abnormalities of mitochondria in muscle tissues. For instance, the presence of ragged-red fibers and abnormal mitochondrial proliferation can be directly observed, confirming the diagnosis of mitochondrial myopathy. Advanced imaging modalities, including magnetic resonance spectroscopy (MRS) and positron emission tomography (PET), are being used to non-invasively assess mitochondrial function and bioenergetics in affected tissues. These imaging techniques help in the evaluation of disease severity and progression, as well as in monitoring the response to therapeutic interventions. The integration of these advanced diagnostic tools into clinical practice is transforming the landscape of mitochondrial myopathy diagnosis, leading to more accurate and timely identification of these disorders, which is essential for effective management and treatment planning.
Personalized Medicine:
The mitochondrial myopathies market is undergoing a transformative shift towards personalized medicine, which aims to tailor treatments to the individual genetic and phenotypic profiles of patients. This approach is particularly relevant for mitochondrial myopathies, given the genetic heterogeneity and complexity of these disorders. Personalized medicine involves using advanced genetic testing to identify specific mutations within the mitochondrial DNA (mtDNA) or nuclear DNA that affect mitochondrial function. For example, whole-genome sequencing (WGS) and whole-exome sequencing (WES) are employed to pinpoint precise genetic anomalies in patients. By understanding the exact genetic defects, clinicians can develop customized therapeutic strategies that target the root cause of the disorder. This precision medicine approach enhances the effectiveness of treatments and minimizes potential side effects, providing a more targeted and patient-centric approach to managing mitochondrial myopathies.
One notable instance of personalized medicine in mitochondrial myopathies is the development of mutation-specific therapies. For example, therapies that target specific mtDNA mutations, such as the use of antisense oligonucleotides (ASOs) to modulate gene expression, are being explored. ASOs can bind to the mutated mRNA transcripts, reducing their harmful effects and improving mitochondrial function. Additionally, the use of tailored pharmacological treatments based on individual genetic profiles is gaining traction. For instance, certain patients with specific genetic mutations may respond better to coenzyme Q10 or other mitochondrial-targeted supplements, which can enhance cellular energy production and alleviate symptoms. Another promising area is the use of personalized gene editing techniques, such as CRISPR/Cas9, to correct specific genetic defects in patient-derived cells. These advancements in personalized medicine not only improve therapeutic outcomes but also offer hope for developing curative treatments for mitochondrial myopathies, transforming the lives of affected individuals and their families.
Buy Full Report: https://www.imarcgroup.com/checkout?id=6999&method=587
Leading Companies in the Mitochondrial Myopathies Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global mitochondrial myopathies market, several leading companies in the mitochondrial myopathies market are at the forefront of developing innovative therapies and diagnostic solutions. Some of the major players include Mitobridge, Reneo Pharmaceuticals, and Stealth BioTherapeutics. These companies are leading the charge in addressing the complex challenges of mitochondrial myopathies, leveraging advanced scientific research and innovative technologies to develop effective treatments and improve patient outcomes.
Mitobridge, a subsidiary of Astellas Pharma, is at the forefront of developing innovative therapies for mitochondrial myopathies. a Phase 1 study demonstrated that bocidelpar was well-tolerated and led to dose-dependent upregulation of PPARδ target genes, indicating enhanced mitochondrial function.
Moreover, Reneo Pharmaceuticals has been making headlines in the mitochondrial myopathies market with its investigational drug, mavodelpar (REN001). Mavodelpar is a potent and selective PPARδ agonist designed to enhance mitochondrial function and address the unmet medical needs of patients with primary mitochondrial myopathies (PMM).
Apart from this, Stealth BioTherapeutics conducts global Phase 3 clinical trials for elamipretide in other indications, such as dry age-related macular degeneration (AMD) and primary mitochondrial myopathy. These trials aim to assess the efficacy and safety of daily subcutaneous injections of elamipretide. Stealth has also entered a licensing agreement with Pharmanovia to commercialize elamipretide for Barth syndrome across Europe, the Middle East, and North Africa, further expanding its reach and impact.
Request for customization: https://www.imarcgroup.com/request?type=report&id=6999&flag=E
Regional Analysis:
The major markets for mitochondrial myopathies include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for mitochondrial myopathies while also representing the biggest market for their treatment. This can be attributed to advancements in diagnostic technologies, increasing awareness, and significant research and development efforts.
Moreover, techniques such as magnetic resonance spectroscopy (MRS) and positron emission tomography (PET) are used to assess mitochondrial function and bioenergetics in affected tissues non-invasively. These imaging modalities are crucial for evaluating disease severity, monitoring progression, and assessing the efficacy of therapeutic interventions.
Besides this, efforts from patient advocacy groups, such as the United Mitochondrial Disease Foundation (UMDF) and the MitoAction, play a crucial role in raising awareness and educating the public about mitochondrial diseases across the country.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2034
Countries Covered
This report offers a comprehensive analysis of current mitochondrial myopathies marketed drugs and late-stage pipeline drugs.
In-Market Drugs
IMARC Group Offer Other Reports:
Healthcare IT Market: The global healthcare IT market size reached US$ 327.9 Billion in 2023, and projected to reach US$ 843.7 Billion by 2032, exhibiting a growth rate (CAGR) of 10.7% during the forecast period from 2024 to 2032.
Transdermal Drug Delivery Systems Market: The global transdermal drug delivery systems market size reached US$ 6.7 Billion in 2023, and projected to reach US$ 11.1 Billion by 2032, exhibiting a growth rate (CAGR) of 5.5% during the forecast period from 2024 to 2032.
Serum-Free Media Market: The global serum-free media market size reached US$ 1.3 Billion in 2023, and projected to reach US$ 2.7 Billion by 2032, exhibiting a growth rate (CAGR) of 8.19% during the forecast period from 2024 to 2032.
Emergency Contraceptive Pills Market: The global emergency contraceptive pills market size reached US$ 585.9 Million in 2023, and projected to reach US$ 814.3 Million by 2032, exhibiting a growth rate (CAGR) of 3.5% during the forecast period from 2024 to 2032.
G-Protein Coupled Receptors (GPCRs) Market: The global G-protein coupled receptors market size reached US$ 3.4 Billion in 2023, and projected to reach US$ 5.7 Billion by 2032, exhibiting a growth rate (CAGR) of 5.69% during the forecast period from 2024 to 2032.
Healthcare RCM Outsourcing Market: The global healthcare RCM outsourcing market size reached US$ 27.8 Billion in 2023, and projected to reach US$ 102.9 Billion by 2032, exhibiting a growth rate (CAGR) of 15.2% during the forecast period from 2024 to 2032.
Digestive Health Products Market: The global digestive health products market size reached US$ 49.0 Billion in 2023, and projected to reach US$ 90.8 Billion by 2032, exhibiting a growth rate (CAGR) of 6.9% during the forecast period from 2024 to 2032.
Healthcare Staffing Market: The global healthcare staffing market size reached US$ 41.8 Billion in 2023, and projected to reach US$ 69.1 Billion by 2032, exhibiting a growth rate (CAGR) of 5.6% during the forecast period from 2024 to 2032.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
The market is driven by increased research and development efforts. Additionally, there is a growing focus on personalized medicine approaches, tailoring treatments to individual genetic profiles, which further propels the market expansion.
Advancements in Gene Therapy: Driving the Mitochondrial Myopathies Market
The mitochondrial myopathies market is witnessing remarkable progress, primarily due to advancements in gene therapy. Gene therapy aims to address the underlying genetic defects causing mitochondrial myopathies, offering hope for more effective and potentially curative treatments. One notable advancement is the development of mitochondrial gene replacement therapy, which involves delivering functional copies of defective genes to patient cells. For instance, the use of adeno-associated virus (AAV) vectors has shown promise in preclinical studies. These vectors can efficiently target and deliver therapeutic genes to mitochondria, improving cellular function. A significant example of this is the gene therapy approach being developed for Leigh syndrome, a severe mitochondrial disorder. Researchers have successfully used AAV vectors to deliver the ND4 gene to affected cells, resulting in improved mitochondrial function and extended survival in animal models.
Request a PDF Sample Report: https://www.imarcgroup.com/mitochondrial-myopathies-market/requestsample
Another critical advancement in gene therapy for mitochondrial myopathies is the use of mitochondrial-targeted RNA therapeutics. This approach involves designing RNA molecules that can selectively bind and correct mutations in mitochondrial DNA (mtDNA). For instance, mitochondrial-targeted RNA import systems have been developed to introduce therapeutic RNA into mitochondria, effectively restoring normal function. A groundbreaking instance is the research on using peptide nucleic acids (PNAs) conjugated with mitochondrial targeting sequences to correct specific point mutations in mtDNA. These PNAs have shown the ability to selectively bind and repair mutated mtDNA, leading to significant functional improvements in cellular and animal models. Additionally, the CRISPR/Cas9 gene-editing technology is being explored for its potential to precisely edit mtDNA mutations, offering a powerful tool for developing targeted therapies. These advancements in gene therapy, coupled with ongoing research and clinical trials, hold great promise for transforming the treatment landscape for mitochondrial myopathies, providing patients with more effective and long-lasting solutions.
Enhanced Diagnostic Techniques: Contributing to Market Expansion
The mitochondrial myopathies market is significantly benefiting from advancements in diagnostic techniques, enabling earlier and more accurate detection of these complex disorders. One of the most notable advancements is the use of next-generation sequencing (NGS) technologies. NGS allows for comprehensive analysis of the entire mitochondrial genome as well as nuclear genes associated with mitochondrial function. This high-throughput technology can identify rare and novel mutations with unprecedented speed and accuracy. For example, whole-exome sequencing (WES) and whole-genome sequencing (WGS) have become invaluable tools in diagnosing mitochondrial myopathies, allowing for the identification of mutations that were previously undetectable with traditional methods. This precise genetic information is crucial for guiding treatment decisions and genetic counseling for affected families.
In addition to NGS, other enhanced diagnostic techniques such as muscle biopsy with histochemical analysis and advanced imaging technologies are improving the accuracy of mitochondrial myopathy diagnoses. Muscle biopsies, when combined with immunohistochemistry and electron microscopy, provide detailed insights into the structural and functional abnormalities of mitochondria in muscle tissues. For instance, the presence of ragged-red fibers and abnormal mitochondrial proliferation can be directly observed, confirming the diagnosis of mitochondrial myopathy. Advanced imaging modalities, including magnetic resonance spectroscopy (MRS) and positron emission tomography (PET), are being used to non-invasively assess mitochondrial function and bioenergetics in affected tissues. These imaging techniques help in the evaluation of disease severity and progression, as well as in monitoring the response to therapeutic interventions. The integration of these advanced diagnostic tools into clinical practice is transforming the landscape of mitochondrial myopathy diagnosis, leading to more accurate and timely identification of these disorders, which is essential for effective management and treatment planning.
Personalized Medicine:
The mitochondrial myopathies market is undergoing a transformative shift towards personalized medicine, which aims to tailor treatments to the individual genetic and phenotypic profiles of patients. This approach is particularly relevant for mitochondrial myopathies, given the genetic heterogeneity and complexity of these disorders. Personalized medicine involves using advanced genetic testing to identify specific mutations within the mitochondrial DNA (mtDNA) or nuclear DNA that affect mitochondrial function. For example, whole-genome sequencing (WGS) and whole-exome sequencing (WES) are employed to pinpoint precise genetic anomalies in patients. By understanding the exact genetic defects, clinicians can develop customized therapeutic strategies that target the root cause of the disorder. This precision medicine approach enhances the effectiveness of treatments and minimizes potential side effects, providing a more targeted and patient-centric approach to managing mitochondrial myopathies.
One notable instance of personalized medicine in mitochondrial myopathies is the development of mutation-specific therapies. For example, therapies that target specific mtDNA mutations, such as the use of antisense oligonucleotides (ASOs) to modulate gene expression, are being explored. ASOs can bind to the mutated mRNA transcripts, reducing their harmful effects and improving mitochondrial function. Additionally, the use of tailored pharmacological treatments based on individual genetic profiles is gaining traction. For instance, certain patients with specific genetic mutations may respond better to coenzyme Q10 or other mitochondrial-targeted supplements, which can enhance cellular energy production and alleviate symptoms. Another promising area is the use of personalized gene editing techniques, such as CRISPR/Cas9, to correct specific genetic defects in patient-derived cells. These advancements in personalized medicine not only improve therapeutic outcomes but also offer hope for developing curative treatments for mitochondrial myopathies, transforming the lives of affected individuals and their families.
Buy Full Report: https://www.imarcgroup.com/checkout?id=6999&method=587
Leading Companies in the Mitochondrial Myopathies Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global mitochondrial myopathies market, several leading companies in the mitochondrial myopathies market are at the forefront of developing innovative therapies and diagnostic solutions. Some of the major players include Mitobridge, Reneo Pharmaceuticals, and Stealth BioTherapeutics. These companies are leading the charge in addressing the complex challenges of mitochondrial myopathies, leveraging advanced scientific research and innovative technologies to develop effective treatments and improve patient outcomes.
Mitobridge, a subsidiary of Astellas Pharma, is at the forefront of developing innovative therapies for mitochondrial myopathies. a Phase 1 study demonstrated that bocidelpar was well-tolerated and led to dose-dependent upregulation of PPARδ target genes, indicating enhanced mitochondrial function.
Moreover, Reneo Pharmaceuticals has been making headlines in the mitochondrial myopathies market with its investigational drug, mavodelpar (REN001). Mavodelpar is a potent and selective PPARδ agonist designed to enhance mitochondrial function and address the unmet medical needs of patients with primary mitochondrial myopathies (PMM).
Apart from this, Stealth BioTherapeutics conducts global Phase 3 clinical trials for elamipretide in other indications, such as dry age-related macular degeneration (AMD) and primary mitochondrial myopathy. These trials aim to assess the efficacy and safety of daily subcutaneous injections of elamipretide. Stealth has also entered a licensing agreement with Pharmanovia to commercialize elamipretide for Barth syndrome across Europe, the Middle East, and North Africa, further expanding its reach and impact.
Request for customization: https://www.imarcgroup.com/request?type=report&id=6999&flag=E
Regional Analysis:
The major markets for mitochondrial myopathies include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for mitochondrial myopathies while also representing the biggest market for their treatment. This can be attributed to advancements in diagnostic technologies, increasing awareness, and significant research and development efforts.
Moreover, techniques such as magnetic resonance spectroscopy (MRS) and positron emission tomography (PET) are used to assess mitochondrial function and bioenergetics in affected tissues non-invasively. These imaging modalities are crucial for evaluating disease severity, monitoring progression, and assessing the efficacy of therapeutic interventions.
Besides this, efforts from patient advocacy groups, such as the United Mitochondrial Disease Foundation (UMDF) and the MitoAction, play a crucial role in raising awareness and educating the public about mitochondrial diseases across the country.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the mitochondrial myopathies market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the mitochondrial myopathies market
- Reimbursement scenario in the market
- In-market and pipeline drugs
This report offers a comprehensive analysis of current mitochondrial myopathies marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
IMARC Group Offer Other Reports:
Healthcare IT Market: The global healthcare IT market size reached US$ 327.9 Billion in 2023, and projected to reach US$ 843.7 Billion by 2032, exhibiting a growth rate (CAGR) of 10.7% during the forecast period from 2024 to 2032.
Transdermal Drug Delivery Systems Market: The global transdermal drug delivery systems market size reached US$ 6.7 Billion in 2023, and projected to reach US$ 11.1 Billion by 2032, exhibiting a growth rate (CAGR) of 5.5% during the forecast period from 2024 to 2032.
Serum-Free Media Market: The global serum-free media market size reached US$ 1.3 Billion in 2023, and projected to reach US$ 2.7 Billion by 2032, exhibiting a growth rate (CAGR) of 8.19% during the forecast period from 2024 to 2032.
Emergency Contraceptive Pills Market: The global emergency contraceptive pills market size reached US$ 585.9 Million in 2023, and projected to reach US$ 814.3 Million by 2032, exhibiting a growth rate (CAGR) of 3.5% during the forecast period from 2024 to 2032.
G-Protein Coupled Receptors (GPCRs) Market: The global G-protein coupled receptors market size reached US$ 3.4 Billion in 2023, and projected to reach US$ 5.7 Billion by 2032, exhibiting a growth rate (CAGR) of 5.69% during the forecast period from 2024 to 2032.
Healthcare RCM Outsourcing Market: The global healthcare RCM outsourcing market size reached US$ 27.8 Billion in 2023, and projected to reach US$ 102.9 Billion by 2032, exhibiting a growth rate (CAGR) of 15.2% during the forecast period from 2024 to 2032.
Digestive Health Products Market: The global digestive health products market size reached US$ 49.0 Billion in 2023, and projected to reach US$ 90.8 Billion by 2032, exhibiting a growth rate (CAGR) of 6.9% during the forecast period from 2024 to 2032.
Healthcare Staffing Market: The global healthcare staffing market size reached US$ 41.8 Billion in 2023, and projected to reach US$ 69.1 Billion by 2032, exhibiting a growth rate (CAGR) of 5.6% during the forecast period from 2024 to 2032.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800