- NEO212™ completes initial two cohorts of Phase 1 study under FDA Investigative New Drug (IND).
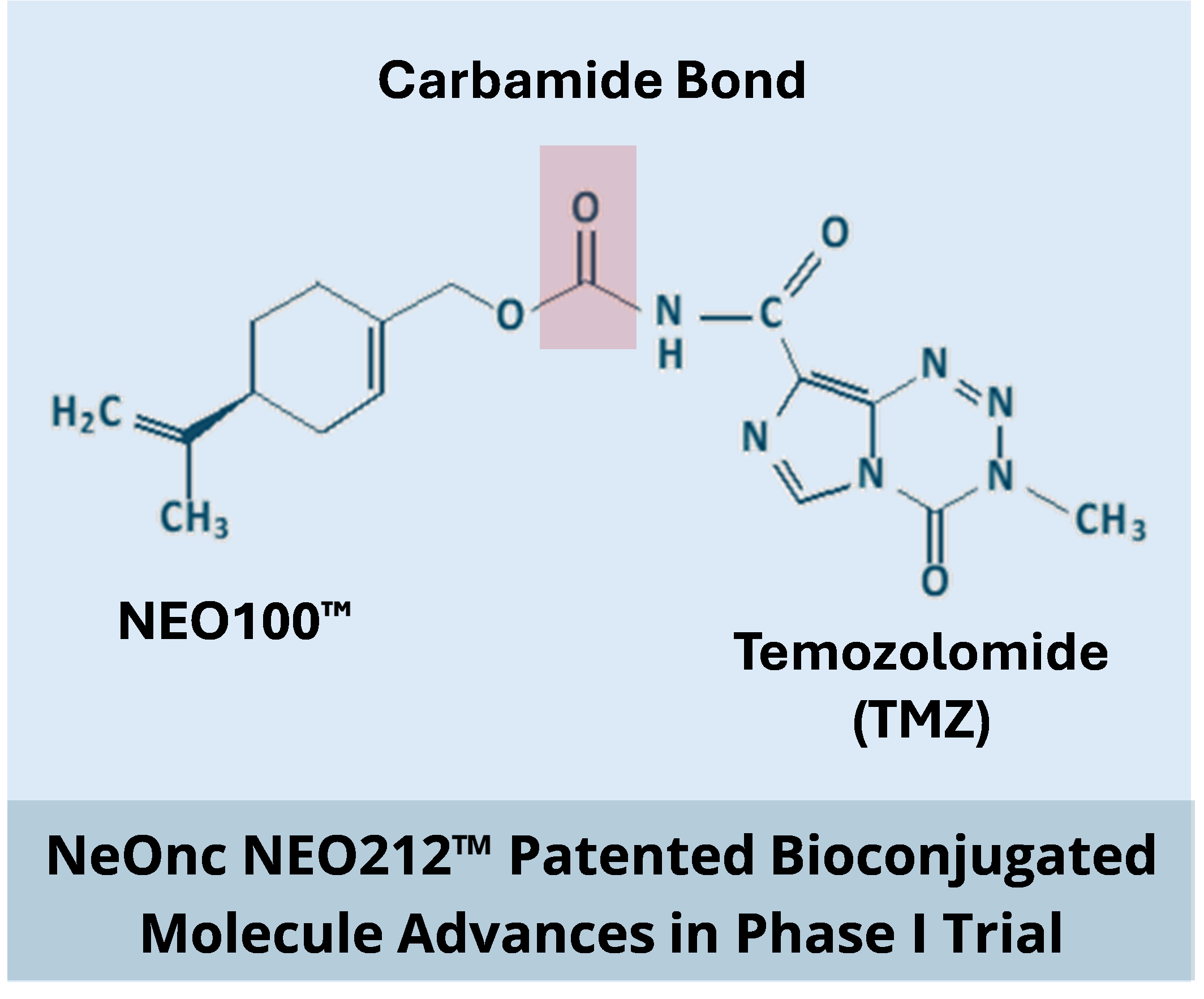
- Study tests the safety of NeOnc’s patented novel drug, NEO212™, which is the bioconjugation of its leading drug candidate NEO100™ and Temozolomide (TMZ).
- As an FDA-approved, off-patent drug under Merck, TMZ is considered the current standard of chemotherapeutic care for patients with Glioblastoma (GBM) brain tumors, with sales exceeding an estimated $1.5 billion last year.
- The covalently bonded bioconjugate NEO212 is designed to deliver more effective brain cancer treatment versus a simple mix of NEO100 and TMZ.
- The NEO™ technology platform enables novel drug production via bioconjugation and unique delivery methods designed to address the persistent challenges with overcoming the blood-brain barrier.
WESTLAKE VILLAGE, Calif., July 24, 2024 (GLOBE NEWSWIRE) -- NeOnc Technologies Holdings, Inc., a clinical-stage medical biotechnology company, has begun enrollment of patients for Cohort 3 out of 6 of the Phase 1 clinical trial of NEO212™, the company’s patented novel hybrid drug designed to deliver a ‘double punch’ against primary and secondary malignant brain tumors.
The advancement to Cohort 3 follows successful completion of the first two cohorts. The company anticipates the final cohort to be completed by the end of December.
The company earlier announced that the FDA authorized NeOnc to initiate Phase 1 and 2 clinical trials of NEO212. The Phase 1 portion of the clinical trial is an open-label 3+3 oral dose escalation of NEO212. The inclusion criteria targets all comers with either primary or secondary solid intracranial tumors.
“Now that we are nearly halfway through our Phase 1 clinical trial of NEO212, we are encouraged that NEO212 may eventually impact the standard of care for primary brain tumors and other cancers that can spread to the brain,” commented NeOnc CEO, Thomas Chen, MD, Ph.D.
NEO212 combines the DNA alkylating agent Temozolomide (TMZ) with NEO100™, NeOnc’s leading drug candidate that is a proprietary, highly purified formulation of Perillyl Alcohol (POH). NEO212 was designed to increase the efficacy of TMZ through oral delivery using the conjugated formulation.
Merck’s patent protection of temozolomide expired in 2009, enabling it to be produced as a generic drug. Sales of TMZ exceeded an estimated $1.5 billion last year.
"Our preclinical animal studies suggest that NEO212 may be less toxic and could potentially penetrate the blood-brain barrier three times more efficiently and with ten times the potency of TMZ—the current standard of chemotherapeutic care,” noted Dr. Chen.
NeOnc's executive chairman, Amir Heshmatpour, added: “We are confident that our clinical trials will validate the result from our pre-clinical research studies conducted by Dr. Chen and our USC Keck Medical School PhD teams, and propel our proprietary drug development and delivery platform toward commercialization.”
NEO212 also addresses brain metastasis, as the most common type of brain tumor, with primary cancers, such as lung, breast and melanoma, most likely metastasizing to the brain.
An estimated 8%-10% of patients with systemic cancers will develop brain metastases representing approximately 200,000 new patients with brain metastases annually, according to the American Association of Neurological Surgeons. For patients with malignant brain tumors, the five-year relative survival rate following diagnosis remains at less than 36%.
These factors are driving the therapeutics market for brain metastasis to grow at a projected 7.8% CAGR to reach $6.9 billion by 2033.
The biotechnology strides generated by NeOnc's NEO™ drug development platform is the result of more than a decade of research at the University of Southern California (USC) by Dr. Chen and his medical and scientific teams.
The NEO212 clinical trial is titled, ‘Open-Label, Phase 1/2 Dose Finding, Safety and Efficacy Study of Oral NEO212 in Patients with Astrocytoma IDH-Mutant, Glioblastoma IDH-Wildtype or Uncontrolled Metastasis to the Brain in Patients with Select Solid Tumors.’
The company recently announced that its Phase 2a clinical trial of NEO100-01™, titled, 'An Open-Label, Phase 1/2a Dose Escalation Study of Safety and Efficacy of NEO100 in Recurrent Grade IV Glioma,’ was expanded from Grade IV to Grade III Astrocytoma with IDH1 mutation.
The clinical trials advance the R&D of NEO™ technology platform and its ability to produce novel drugs and delivery methods designed to address the persistent challenges with overcoming the blood-brain barrier.
About NeOnc Technologies Holdings
NeOnc Technologies is a privately held clinical stage life sciences company focused on the development and commercialization of central nervous system therapeutics that are designed to address the persistent challenges in overcoming the blood-brain barrier.
The company’s NEO™ drug development platform has produced a portfolio of novel drug candidates and delivery methods with patent protections extending to 2038. These proprietary chemotherapy agents have demonstrated positive effects in laboratory tests on various types of cancers and in clinical trials treating malignant gliomas. NeOnc’s NEO100™ and NEO212™ therapeutics are in Phase I and II human clinical trials and are advancing under FDA Fast-Track and Investigational New Drug (IND) status.
The company has exclusively licensed an extensive worldwide patent portfolio from the University of Southern California consisting of issued patents and pending applications related to NEO100, NEO212, and other products from the NeOnc patent family for multiple uses, including oncological and neurological conditions.
For more about NeOnc and its pioneering technology, visit neonctech.com.
Important Cautions Regarding Forward-Looking Statements
All statements other than statements of historical facts included in this press release are "forward-looking statements" (as defined in the Private Securities Litigation Reform Act of 1995). Generally, such forward-looking statements include statements regarding our expectations, possible or assumed future actions, business strategies, events, or results of operations, including statements regarding our expectations or predictions or future financial or business performance or conditions and those statements that use forward-looking words such as "projected," "expect," "possibility" and "anticipate," or similar expressions. The achievement or success of the matters covered by such forward-looking statements involve significant risks, uncertainties, and assumptions. Actual results could differ materially from current projections or implied results.
NeOnc Technologies Holding, Inc. (the "Company") cautions that statements and assumptions made in this news release constitute forward-looking statements without guaranteeing future performance. Forward-looking statements are based on estimates and opinions of management at the time statements are made. The information set forth herein speaks only as of the date hereof. The Company and its management are under no obligation, and expressly disclaim any responsibility, to update, alter, or otherwise revise any forward-looking statements following the date of this news release, whether because of new information, future events, or otherwise, except as required by law.
NeOnc Company Contact:
Patrick Walters
COO
NeOnc Technologies Holdings, Inc.
Email Contact
NeOnc Investor Relations:
Ron Both or Grant Stude
CMA Investor Relations
Tel (949) 432-7566
Email Contact
NeOnc Media & ESG Contact:
Tim Randall
CMA Media Relations
Tel (949) 432-7572
Email Contact
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/c231e557-8b1e-4e68-8ecf-d0d97fc4c93e





