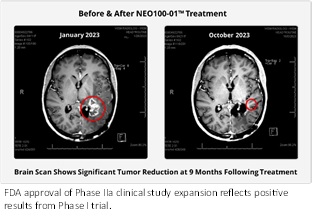
- FDA approval reflects positive results from Phase I and preliminary results from Phase IIa study currently underway that demonstrate increased survivability rates in patients with the IDH1 marker.
- Trial expands from patients afflicted with Recurrent Grade IV Astrocytoma with IDH1 mutation to those with equally aggressive Recurrent Grade III Astrocytoma with IDH1 mutation.
- Enrollment in three other NeOnc clinical studies also advance after several published studies indicate the viability of NeOnc’s NEO™ platform to produce drugs which can function as therapeutics or blood-brain barrier delivery vehicles for chemotherapeutics that can beneficially effect brain cancer treatment.
WESTLAKE VILLAGE, Calif., July 16, 2024 (GLOBE NEWSWIRE) -- NeOnc Technologies Holdings, Inc., a clinical-stage medical biotechnology company, received FDA approval to expand its ongoing NEO100-01™ Phase 2a clinical trial to include Recurrent Grade III Astrocytoma with Isocitrate Dehydrogenase 1 (IDH1) mutation.
The FDA approval expands the current clinical trial, “An Open-Label, Phase 1/2a Dose Escalation Study of Safety and Efficacy of NEO100 in Recurrent Grade IV Glioma,” from only Recurrent Grade IV tumors that more often affects senior adults (typically 50-80 years of age) to a younger patient population (typically ages 30-40) that are more often affected by Grade III.
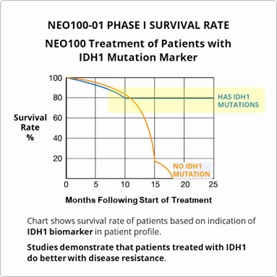
NeOnc looks to build upon the published results from its Phase 1 clinical safety study which demonstrated greater survival rates with NEO100-01, and particularly with patients who received additional treatments cycles and those with the IDH1 marker, as well as against current standard-of-care treatments.
“This significant approval advances the research and development of our NEO™ technology platform and its ability to produce novel drugs and delivery methods designed to address the persistent challenges with overcoming the blood-brain barrier,” stated NeOnc CEO and founder, Thomas Chen, MD, Ph.D. “Moreover, it opens the field for us to investigate how NEO100-01 could be safe and efficacious for younger populations before their cancers potentially worsen to Grade IV.”
An estimated 1 million Americans are living with a primary brain tumor, with an estimated 26,940 new malignant tumor cases diagnosed in the U.S. last year, according to the National Brain Tumor Society. For patients with malignant brain tumors, the five-year relative survival rate following diagnosis remains at less than 36%.
Recurrent Grade III Astrocytoma with IDH1 mutations are also known to manifest as aggressively as its Grade IV/IDH1 counterparts, which further highlights the importance of the study expansion.
The biotechnology strides generated by NeOnc’s patented NEO platform are the result of more than a decade of research at the University of Southern California (USC) by Dr. Chen and his medical and scientific teams. Dr. Chen has been leading the company’s Phase I and II clinical trials.
According to NeOnc executive chairman, Amir Heshmatpour: “This expansion of NEO100’s applicability truly marks a monumental step forward for NeOnc Technologies. By broadening the inclusion criteria to encompass patients with Recurrent Grade III Astrocytoma with the IDH1 mutation, we not only advance our innovative platform but also provide new hope for patients who previously had limited treatment options.”
“Our drive to push the boundaries of brain cancer treatment has never been stronger,” added Heshmatpour, “and this trial expansion is a testament to our team’s dedication to transforming patient outcomes.”
By enabling better drug delivery to the brain cancer, the company believes its NEO technology can turn existing FDA-approved drugs into more effective treatments across a full range of central nervous system (CNS) disorders. The company is developing several additional proprietary chemotherapy agents that have demonstrated positive effects in laboratory tests on other various types of cancers.
“As we continue to consult with the FDA under its Orphan Drug and Fast-Track status, we expect to collect sufficient data that demonstrates the important therapeutic value of our four leading drug candidates,” added Dr. Chen. “We believe our novel intranasal delivery approach will enable studies in difficult groups, including the pediatric population.”
“Radiation and chemotherapy, especially for children and senior adults with high-grade gliomas, is complex, time consuming and prognosis remains poor,” added Dr. Chen. “This underscores the importance of us developing effective therapies that are less invasive and more tolerable for challenged populations.”
About Astrocytoma Category Types
The World Health Organization categorizes Astrocytoma into four grades. The grades depend on how fast the Astrocytoma grows and the likelihood that it will spread to or infiltrate nearby brain tissue.
Grade I Astrocytoma is the mildest form of brain cancer which most often affects children and teens.
Grade II Astrocytoma most often affect adults between the ages 20 and 60. These types of cancers tend to spread to nearby brain tissue, so surgery alone might not be enough to treat them.
Grade III Astrocytoma most often affect adults between 30 and 60 years of age. Grade III often presents as a progression from Grade II and is more aggressive. Surgery alone never cures these tumors; they require radiation and almost always require chemotherapy.
Grade IV Astrocytoma, also known as Glioblastomas, are the most common type of brain cancer in adults and account for 24% of all brain tumors. They are also the most dangerous and aggressive type of Astrocytoma and are known to spread quickly. They can either present as a cancerous progression from a previously existing lower-grade Astrocytoma (10% of cases) or begin as a Grade IV tumor (90% of cases).
About NeOnc Technologies Holdings
NeOnc Technologies is a privately held clinical stage life sciences company focused on the development and commercialization of central nervous system therapeutics that are designed to address the persistent challenges in overcoming the blood-brain barrier.
The company’s NEO™ drug development platform has produced a portfolio of novel drug candidates and delivery methods with patent protections extending to 2038. These proprietary chemotherapy agents have demonstrated positive effects in laboratory tests on various types of cancers and in clinical trials treating malignant gliomas. NeOnc’s NEO100™ and NEO212™ therapeutics are in Phase I and II human clinical trials, and are advancing under FDA Fast-Track and Investigational New Drug (IND) status.
The company has exclusively licensed an extensive worldwide patent portfolio from the University of Southern California consisting of issued patents and pending applications related to NEO100, NEO212, and other products from the NeOnc patent family for multiple uses, including oncological and neurological conditions.
For more about NeOnc and its pioneering technology, visit neonctech.com.
Important Cautions Regarding Forward-Looking Statements
All statements other than statements of historical facts included in this press release are “forward-looking statements” (as defined in the Private Securities Litigation Reform Act of 1995). Generally, such forward-looking statements include statements regarding our expectations, possible or assumed future actions, business strategies, events, or results of operations, including statements regarding our expectations or predictions or future financial or business performance or conditions and those statements that use forward-looking words such as “projected,” “expect,” “possibility” and “anticipate,” or similar expressions. The achievement or success of the matters covered by such forward-looking statements involve significant risks, uncertainties, and assumptions. Actual results could differ materially from current projections or implied results.
NeOnc Technologies Holding, Inc. (the “Company”) cautions that statements and assumptions made in this news release constitute forward-looking statements without guaranteeing future performance. Forward-looking statements are based on estimates and opinions of management at the time statements are made. The information set forth herein speaks only as of the date hereof. The Company and its management are under no obligation, and expressly disclaim any responsibility, to update, alter, or otherwise revise any forward-looking statements following the date of this news release, whether because of new information, future events, or otherwise, except as required by law.
NeOnc Company Contact:
Patrick Walters
COO
NeOnc Technologies Holdings, Inc.
Email Contact
NeOnc Investor Relations:
Ron Both or Grant Stude
CMA Investor Relations
Tel (949) 432-7566
Email Contact
NeOnc Media & ESG Contact:
Tim Randall
CMA Media Relations
Tel (949) 432-7572
Email Contact
A photo accompanying this announcement is available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/4086a689-17b2-4681-af7c-f5a84afe3011
https://www.globenewswire.com/NewsRoom/AttachmentNg/31dc10bf-6fb9-4e26-b16f-b37eac3ca0fc





