Neurocrine Biosciences, Inc. (Nasdaq: NBIX) today announced that the primary study results from its CAHtalyst™ Phase 3 study investigating crinecerfont for the treatment of adults ages 18 and older with congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency have been published in The New England Journal of Medicine online edition and will appear in a future print issue of the journal.
|
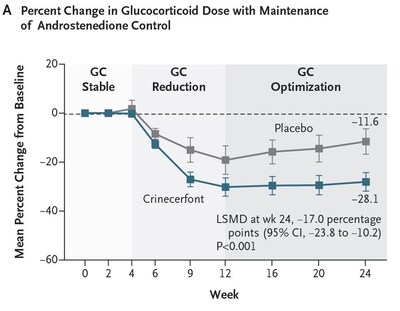
- CAHtalyst™ Adult Phase 3 Study Met Primary and Important Key Secondary Endpoints, with Crinecerfont Treatment Decreasing Androstenedione Levels and Enabling Glucocorticoid Dose Reduction While Maintaining Androstenedione Control SAN DIEGO, June 3, 2024 /PRNewswire/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) today announced that the primary study results from its CAHtalyst™ Phase 3 study investigating crinecerfont for the treatment of adults ages 18 and older with congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency have been published in The New England Journal of Medicine online edition and will appear in a future print issue of the journal. The study met the primary and important key secondary endpoints related to androgen reduction (during an initial glucocorticoid-stable period) and glucocorticoid (GC) dose reduction while maintaining androgen control. Favorable trends were observed with endpoints that reflect the consequences of long-term supraphysiologic glucocorticoid therapy. In addition to being published in The New England Journal of Medicine, CAHtalyst Adult Phase 3 data were presented at ENDO 2024 in an oral presentation by Dr. Richard Auchus. "Since the 1950s, glucocorticoids have been required not only for cortisol replacement, but also to control the excessive amount of adrenal androgens in patients with congenital adrenal hyperplasia. As a result, CAH patients suffer from a higher prevalence of disorders attributable to supraphysiologic GC levels, including osteoporosis, obesity, insulin resistance, diabetes mellitus, hyperlipidemia and hypertension," said Dr. Richard Auchus, M.D., Ph.D., Principal Investigator, Professor of Pharmacology and Internal Medicine, Division of Metabolism, Endocrinology, and Diabetes at the University of Michigan. "The CAHtalyst Adult Phase 3 study demonstrated that crinecerfont achieved a significantly greater GC reduction from Week 4 through Week 24 than placebo while androstenedione was maintained and did so safely. Crinecerfont could be the treatment alternative that we've needed to effectively treat our CAH patients while mitigating the comorbidities found with the existing treatment paradigm." Crinecerfont is an investigational, oral, selective corticotropin-releasing factor type 1 receptor (CRF1) antagonist being developed to reduce and control excess adrenocorticotropic hormone (ACTH) and adrenal androgens through a GC-independent mechanism for the treatment of CAH. "The CAHtalyst Adult Phase 3 study demonstrated the ability of crinecerfont to substantially reduce glucocorticoid doses to more physiologic levels while maintaining or improving androgen control," said Eiry W. Roberts, M.D., Chief Medical Officer at Neurocrine Biosciences. "This represents a potential paradigm shift in treatment for adult patients living with this disorder over many years, who consistently struggle with the clinical consequences of poor androgen control and/or excess glucocorticoid caused by current inadequate treatment approaches." The CAHtalyst Adult Phase 3 global registrational study was conducted in 182 participants and designed to evaluate the safety, efficacy, and tolerability of crinecerfont in adults ages 18 years and older with CAH due to 21-hydroxylase deficiency. Over 95% of participants completed the 24-week double-blind, placebo-controlled treatment period of the study with minimal missing data. The Phase 3 Adult study met the primary endpoint of percent change from baseline in GC dose (while maintaining androstenedione control) and the key secondary endpoint of achievement of reduction to a physiologic GC dose (while maintaining androstenedione control) at Week 24.
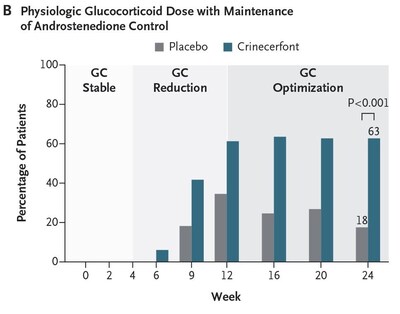
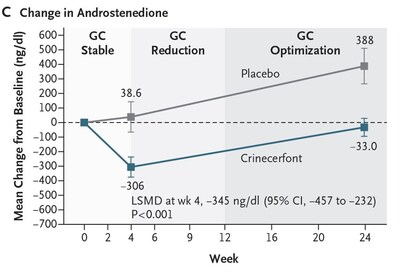
Importantly, a significantly greater proportion of participants treated with crinecerfont achieved reduction to a physiologic GC dose (≤ 11 mg/m2/day) while maintaining androstenedione control compared to placebo (62.7% vs. 17.5%; P < 0.001). Actual value is P < 0.0001. (Figure B) The Phase 3 Adult study also met the key secondary endpoint of change from baseline in androstenedione (key adrenal androgen) following the initial 4-week GC stable period:
At Week 24, mean androstenedione levels remained below baseline with crinecerfont in the context of substantially reduced GC dose. However, androstenedione levels increased to above baseline with placebo, highlighting the inability to maintain androgen control when GC was reduced in the absence of any other intervention. (Figure C) Despite the short-term duration of this Phase 3 study, favorable trends were observed for endpoints associated with long-term supraphysiologic GC therapy such as body weight, insulin resistance, and glucose tolerance that were encouraging considering the relatively short time frame of reduced GC dose. In addition, bone resorption and formation markers increased more with crinecerfont, reflecting relief from GC-induced suppression of bone turnover. Crinecerfont was generally well tolerated, with the most common adverse events being fatigue and headache. Few serious adverse events occurred, with none assessed as related to crinecerfont, and there were few discontinuations due to adverse events. There was no safety concern related to adrenal crisis, with few events reported during the double-blind period and similar incidence of adverse events leading to glucocorticoid stress dosing in the crinecerfont and placebo groups. Note: P-values in the text and figures reflect the Journal's convention of calculating to two significant figures. Actual P-values to three significant figures are also provided. Figures reflect observed means rather than the LSM and LSMD described in the text. Figures are from The New England Journal of Medicine, Phase 3 Trial of Crinecerfont in Adult Congenital Adrenal Hyperplasia, Auchus R, Hamidi O, Pivonello R, et.al. This article was published online on June 1, 2024. Copyright © 2024 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. About Congenital Adrenal Hyperplasia Glucocorticoids (GCs) are currently used not only to correct the endogenous cortisol deficiency, but doses used are higher than cortisol replacement needed (supraphysiologic) to lower the levels of adrenocorticotropic hormone (ACTH) and adrenal androgens. However, glucocorticoid treatment at supraphysiologic doses has been associated with serious and significant complications of steroid excess, including metabolic issues such as weight gain and diabetes, cardiovascular disease, and osteoporosis. Additionally, long-term treatment with supraphysiologic GC doses may have psychological and cognitive impact, such as changes in mood and memory. Adrenal androgen excess has been associated with abnormal bone growth and development in pediatric patients, female health problems such as acne, excess hair growth and menstrual irregularities, testicular rest tumors in males, and fertility issues in both sexes. About Crinecerfont and the CAHtalyst™ Studies The CAHtalyst™ Pediatric and Adult Phase 3 global registrational studies are designed to evaluate the safety, efficacy, and tolerability of crinecerfont in children and adolescents, and adults respectively, with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. The primary portions of the CAHtalyst Phase 3 studies have completed and enrollment is closed, while the open-label treatment portions of both studies are ongoing. Data from the CAHtalyst Pediatric and CAHtalyst Adult Phase 3 studies supported two New Drug Application submissions to the U.S. Food and Drug Administration in April 2024. To learn more about crinecerfont and the CAHtalyst studies, click here. About Neurocrine Biosciences, Inc. The NEUROCRINE BIOSCIENCES Logo Lockup and YOU DESERVE BRAVE SCIENCE are registered trademarks of Neurocrine Biosciences, Inc. CAHtalyst is a trademark of Neurocrine Biosciences, Inc. Forward-Looking Statements
SOURCE Neurocrine Biosciences, Inc. |
||
Company Codes: NASDAQ-NMS:NBIX |