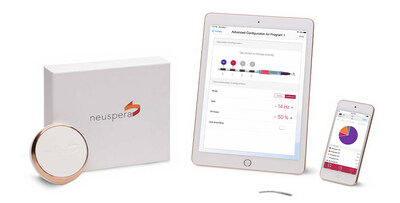
Neuspera® Medical, a medical device company developing implantable devices for patients battling chronic illnesses, today announced it received U.S. Food and Drug Administration (FDA) clearance for the next-generation Neuspera ultra-miniaturized system.
|
First-of-its-kind, leadless micro-implant for chronic peripheral nerve pain management SAN JOSE, Calif., April 18, 2023 /PRNewswire/ -- NeuSpera Medical, a medical device company developing implantable devices for patients battling chronic illnesses, today announced it received U.S. Food and Drug Administration (FDA) clearance for the next-generation Neuspera ultra-miniaturized system. The system is comprised of a micro-implant that delivers neurostimulation therapy through a wireless platform including a wearable transmitter and iPad-based clinician programmer. The Neuspera system delivers peripheral nerve stimulation (PNS) through a wireless, less invasive, and more versatile platform than commercially available technology. It is the first PNS device that offers an ultra-miniaturized option, which may allow for a better patient experience and greater procedural flexibility. It is 75x smaller than the smallest commercial implantable pulse generator and provides physicians the opportunity for deeper anatomical targets compared to current technologies available today. "We look forward to bringing this innovative technology to physicians and patients in the U.S.," commented Steffen Hovard, CEO of Neuspera Medical. "The Neuspera ultra-miniaturized system has the potential to revolutionize the way physicians treat patients battling chronic pain while restoring patients' health and quality of life." In recent years, the growth of PNS technology and treatment has been significant, with advances in implantable device technology and an increasing number of clinical studies focused on developing new PNS therapies. PNS is becoming an increasingly important treatment option for managing chronic pain conditions, with the market expecting to experience significant growth due to factors including the increasing prevalence of chronic pain, advances in implantable device technology, and rising demand for alternative pain management therapies. About Neuspera Medical Investor Relations and Media Contacts
SOURCE Neuspera Medical Inc. |