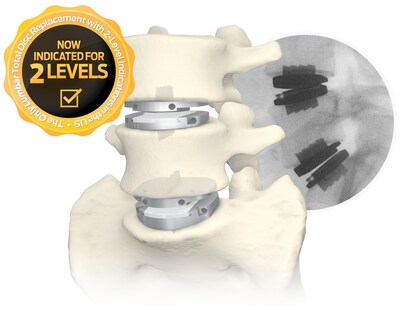
Centinel Spine®, LLC announced that effective January 1, 2023, providers may begin utilizing a new Current Procedural Terminology code when performing two-level lumbar total disc arthroplasty via an anterior approach.
- The American Medical Association has issued a new CPT® add-on code for the second level of lumbar total disc replacement (TDR) procedures
- Centinel Spine continues to advance lumbar motion preservation with focus on both clinical and product development initiatives, as this announcement follows two-level FDA approval and new Anatomic Endplate implant introductions
- Centinel Spine's prodisc® L is the only total disc replacement system in the U.S. approved for two-level use in the lumbar spine
WEST CHESTER, Pa., Jan. 18, 2023 /PRNewswire/ -- Centinel Spine®, LLC, ("the Company") a leading global medical device company addressing cervical and lumbar spinal disease through anterior surgical access, today announced that effective January 1, 2023, providers may begin utilizing a new Current Procedural Terminology (CPT®) code when performing two-level lumbar total disc arthroplasty via an anterior approach. To increase the availability of two-level lumbar total disc replacement (TDR) to individuals experiencing degeneration of the intervertebral discs, the American Medical Association (AMA) accepted the addition of a new Category I CPT code for a second level of lumbar TDR in late 2021. Centinel Spine's prodisc® L is the only total disc replacement system in the U.S. approved for two-level use in the lumbar spine.
New CPT codes are created after review by the AMA CPT Editorial Panel and minimally meet several criteria, including:
- The device is FDA approved.
- The procedure is performed by many physicians across the United States.
- The procedure is performed with frequency consistent with the intended clinical use (i.e., a service for a common condition should have high volume).
- The procedure is consistent with current medical practice.
- The clinical efficacy of the procedure is documented in literature that meets the requirements set forth in the CPT code-change application.
The Editorial Panel consists of a cross-functional membership of medical specialty societies; the Blue Cross and Blue Shield Association; and others that represent America's Health Insurance Plans (AHIP) and private health organizations. The CPT codes created and maintained by the AMA CPT Editorial Panel are widely used by third party payors, including Medicare, Medicaid, and commercial health plans.
"This new CPT code will allow more patients access to two-level lumbar total disc replacement," commented Dr. Vivek Mohan, MD from the Orthopaedic Spine Institute, Schaumburg, IL. "There is a significant number of patients with symptomatic two-level Degenerative Disc Disease in the lumbar spine, and this will impact their ability to benefit from the advantages of lumbar disc replacements."
Centinel Spine CEO Steve Murray further notes, "Through the efforts of physician partners and medical societies, this new CPT code expands access to life changing technologies for patients by opening new reimbursement pathways. The prodisc L device is the only disc that has FDA approval for two-level indications and has been a benefit for patients with lumbar disc disease of this severity. Having two-level approval has helped Centinel pursue its mission to provide clinically-proven products that allow individuals to continue to function at a high level."
Centinel Spine continues to innovate in the lumbar total disc replacement market, with several recent significant prodisc L system achievements, including FDA approval of two-level lumbar indications, the launch of Anatomic Endplates designed to shift the lordotic angle of the lumbar implant to the inferior endplate, and two recent Orthopedics This Week Technology Awards.
Centinel Spine®, LLC is a leading global medical device company addressing cervical and lumbar spinal disease through anterior surgical access. The company offers a continuum of trusted, brand-name, motion-preserving and fusion solutions backed by over 30 years of clinical success—providing the most robust and clinically-proven technology platforms in the world for total disc replacement (prodisc®) and Integrated Interbody™ fusion (STALIF®).
Centinel Spine continues to advance its pioneering culture and corporate mission to become a catalyst of change in the spine industry and alter the way spine surgery is perceived. Centinel Spine remains the only company with comprehensive motion-preserving and fusion solutions for both cervical and lumbar anterior column reconstruction.
For more information, please visit the company's website at www.CentinelSpine.com or contact:
Varun Gandhi
Chief Financial Officer
900 Airport Road, Suite 3B
West Chester, PA 19380
Phone: 484-887-8871
Email: v.gandhi@centinelspine.com
Media
Kyle Evans
ICR Westwicke
Phone: 646-677-1295
Email: kyle.evans@westwicke.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/new-cpt-code-now-effective-for-second-level-of-lumbar-total-disc-replacement-procedures-301724028.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/new-cpt-code-now-effective-for-second-level-of-lumbar-total-disc-replacement-procedures-301724028.html
SOURCE Centinel Spine, LLC