Gladstone scientists discover how the novel coronavirus damages heart muscle cells, and creates a system that can be used to screen potential therapies
|
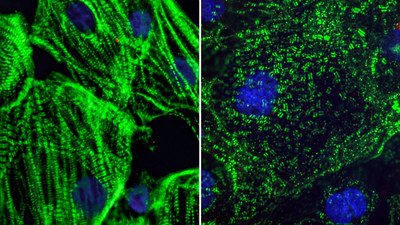
SAN FRANCISCO, Aug. 26, 2020 /PRNewswire/ -- COVID-19 was initially identified as a respiratory disease, but scientists now appreciate that it also affects several other organs in the body, including the heart. Heart damage is a major determinant of COVID-19 related deaths, and even patients who experience only mild COVID-19 symptoms exhibit signs of cardiac dysfunction several months after recovery. A new study by scientists at Gladstone Institutes helps explain how SARS-CoV-2, the virus that causes COVID-19, inflicts damage on heart cells. The team’s findings, shared publicly on bioRxiv, show the virus's unexpected effects on the structure of heart cells in the lab, as well as in heart tissue from COVID-19 patients. The team, led by Gladstone Senior Investigators Todd C. McDevitt, PhD, and Bruce R. Conklin, MD, was uniquely positioned to tackle this work, due to their experience in deriving various types of cardiac cells in the lab from induced pluripotent stem cells. In collaboration with Director of the Gladstone Institute of Virology Melanie Ott, MD, PhD, they exposed the cells to varying doses of SARS-CoV-2. The virus only productively infected the cardiomyocytes, or heart muscle cells, meaning that the virus could enter those cells and make new copies of itself. "Early on in our experiments, we noticed that many of the cardiomyocytes were exhibiting some very strange features," says McDevitt, who is also a professor of bioengineering and therapeutic sciences at UC San Francisco (UCSF). "What we were seeing was completely abnormal; in my years of looking at cardiomyocytes, I had never seen anything like it before." The team observed that when they exposed cardiomyocytes to SARS-CoV-2, the sarcomeres in some of the cells appeared to be diced into small, regularly sized fragments. Typically, sarcomeres—units of the muscle fibers in heart cells—are organized into long filaments aligned in the same direction. These sarcomeres control the coordinated contraction of heart cells to produce the normal heartbeat. "The sarcomere disruptions we discovered would make it impossible for the heart muscle cells to beat properly," explains Conklin, who is also a professor of medicine, cellular and molecular pharmacology, and ophthalmology at UCSF. The scientists also noted that the nuclear DNA seemed to be missing from many of the heart cells. Without DNA, cells can no longer perform any normal functions. "It's the cell equivalent of being brain dead," adds Conklin. "Even after scouring scientific literature and conferring with colleagues, we cannot find these abnormal cell features in any other cardiac disease model. We believe they are unique to SARS-CoV-2 and could explain the prolonged heart damage seen in many COVID-19 patients." Discoveries in a Dish Predict Changes in Human Tissue To understand whether these changes to cells in culture were relevant to COVID-19 in humans, the researchers sought out heart tissue from COVID-19 patients. However, patient tissue was hard to find. "Most people don't appreciate how difficult it has been to access patient samples," says McDevitt. "Since this virus is so contagious, many hospitals lack the special equipment they need to safely perform autopsies on COVID-19 patients." When the team was able to receive patient samples, what they saw corroborated the structural changes they saw in the lab. Remarkably, even in patients who had not been diagnosed with COVID-19 related heart disease, there was evidence of structural abnormalities in the heart muscle cells. Additional testing needs to be done to validate these findings further, but the immediate similarities are striking. "These abnormalities haven't been identified in patients before, so they may have been overlooked," says McDevitt. "I hope our work motivates doctors to review their patients' samples to start looking for these features at a higher magnification, which will be the true test of our hypotheses." Heart Cells Can Serve as a New Drug Screen In addition to providing more insight into the impact of COVID-19 on the heart, the team's model could be used as a novel way to test and screen drugs that could help mitigate the effects of COVID-19 in the heart, as well as other tissues susceptive to SARS-CoV-2 infection. "Heart muscle cells are highly infectible, and we have very visually distinct signs of infection," says Conklin. "Since we can easily see the effects of infection, we can use these cells as a first screen to find drugs that could shut down the virus or prevent it from taking over the cells." Long-term Consequences for COVID-19 Patients Moreover, these findings shed light on the long-term ramifications of COVID-19. Unlike some other tissues in the body, the heart does not regenerate. So, it's possible that someone who becomes infected with COVID-19, even a mild case, could recover and then develop heart disease years later. "It will be important to identify a protective therapy, one that safeguards the heart from the damage we're seeing in our models," says McDevitt. "Even if you can't prevent the virus from infecting cells, you could put a patient on a drug to prevent these negative consequences from occurring while the disease is present." About the Research Project The paper, “SARS-CoV-2 infection of iPSC-derived cardiac cells predicts novel cytopathic features in COVID-19 patients,” was shared publicly on bioXriv on August 25, 2020. Other authors include Juan A. Pérez-Bermejo, Serah Kang, Sarah J. Rockwood, Ana C. Silva, Huihui Li, and Ken Nakamura, all of Gladstone Institutes; David A. Joy, Will R. Flanigan, Camille R. Simoneau, and Gokul N. Ramadoss, all graduate students at UCSF, training in Gladstone Institutes' labs; and Jeffrey D. Whitman, Laboratory Medicine, UCSF. The work and researchers involved were supported by grants from the National Institutes of Health (R01-HL130533, R01-HL13535801, P01-HL146366, and U01 ES032673-03), the National Science Foundation (NSF ERC 1648035), and gifts from the Roddenberry Foundation and Tom and Pauline Tusher. About Gladstone Institutes To ensure our work does the greatest good, Gladstone Institutes focuses on conditions with profound medical, economic, and social impact—unsolved diseases. Gladstone is an independent, nonprofit life science research organization that uses visionary science and technology to overcome disease. Media Contact: Julie Langelier | Assistant Director, Communications | julie.langelier@gladstone.org | 415.734.5000
SOURCE Gladstone Institutes |