New research reveals that precision prescribing using genetic testing has finally reached a global tipping point for adoption.
|
Life science leaders say the time is finally right for healthcare systems, employers, and governments to establish genome-informed clinical decision-making as the new standard of care PHILADELPHIA, Jan. 18, 2023 /PRNewswire/ -- The Human Genome Project, completed nearly 20 years ago, promised an era of personalized healthcare. Now, new research reveals that precision prescribing using genetic testing has finally reached a global tipping point for adoption. "We're now entering a new age where barriers have transformed into boons for PGx-enabled precision medicine."
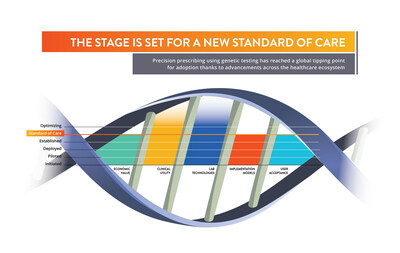
Published by Cambridge University Press, the peer-reviewed research—authored by life science leaders at Coriell Life Sciences—examines the maturation of critical factors required for the use of clinical genetics at scale. These advancements pave the way in enabling genome-informed clinical decision-making to be established as the new standard of care. "The stage is set for leaders around the world to change healthcare as we know it," says co-author Scott Megill, President and CEO of Coriell Life Sciences, an international leader in precision medicine. "Studies have shown that using genetic information to provide more targeted therapeutic care significantly improves patient outcomes and reduces the overall cost of healthcare. This is especially true for pharmacogenomics (PGx), which uses an individual's DNA to determine if medications are likely to be unsafe or ineffective for them specifically. We're now entering a new age where barriers have transformed into boons for PGx-enabled precision medicine." The research tracks and analyzes global readiness for the large-sale implementation of PGx-enabled precision medicine based on five key factors:
The paper also explores other important factors fueling readiness for PGx, including:
The paper emphasizes that, "Genetically informed prescribing offers one of the few realistic mechanisms to effectively reduce overall healthcare system costs while also improving individual patient care and outcomes." The authors conclude, "The time is now for healthcare systems, employers, and governments to partner and establish genome-informed clinical decision-making as the new standard of care." These findings are supported by the Personalized Medicine Coalition (PMC), a group representing 220 member organizations comprised of innovators, scientists, patients, providers, and payers. PMC promotes the understanding and adoption of personalized medicine concepts, services, and products to benefit patients and health systems. "This research details significant industry progress toward the clinical utilization of pharmacogenomics and personalized medicine on a population scale. Thanks to these advancements, which represent the work of multiple stakeholders, a new standard of care is finally within reach," says Daryl Pritchard, Ph.D., Senior Vice President, Science Policy, Personalized Medicine Coalition. Jay G. Wohlgemuth, M.D., Board Chair of the Personalized Medicine Coalition and SVP R&D and Chief Medical Officer for Quest Diagnostics, adds, "Pharmacogenomics provides insights, based on a patient's unique genetics, to help identify the best therapy in advance, reducing the risk of adverse side effects and wasted time and dollars on an ineffectual treatment. As additional research shows, when delivered as part of a consumer-centric medication management program that includes informatics and pharmacy services, pharmacogenomics is scalable to large populations and can deliver actionable insights for a majority of individuals. The field has matured to a stage where the many payers of healthcare services—including employers and health plans—can confidently embrace pharmacogenomics in their population health programs to optimize medical spend and outcomes." To learn more, read the full paper, "Maturing pharmacogenomics factors deliver improvements and cost efficiencies." About Coriell Life Sciences
Coriell Life Sciences (CLS) uses innovation in precision medicine to reduce healthcare costs and empower a healthier world. With scientific expertise that spans six decades, CLS bridges the gap between genetic knowledge and clinical application and offers the most comprehensive medication risk management program on the market. Visit coriell.com. Media Contact:
SOURCE Coriell Life Sciences |