Quadrant Laboratories announced that its Syracuse-based lab has been approved by the New York State Department of Health for genetic testing.
Quadrant Laboratories' Fragile X Syndrome test is now approved by the New York State Department of Health and is available in all 50 states
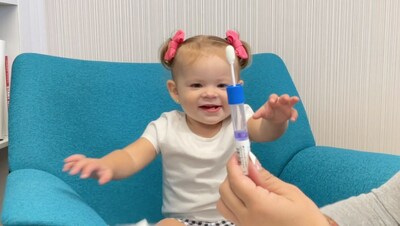
SYRACUSE, N.Y., Oct. 17, 2023 /PRNewswire/ -- Quadrant Laboratories announced today that its Syracuse-based lab has been approved by the New York State Department of Health for genetic testing. This is in conjunction with the NYSDOH approval of Quadrant's Fragile X Syndrome (FXS) test, which is now available for physicians to order in all 50 states. Quadrant's FXS test uses a saliva swab as a primary collection, rather than a blood draw, and can be collected in the patient's home before getting shipped back to be tested in Quadrant's CLIA/CLEP certified clinical lab.
Dr. Funda Suer, EVP of Clinical Diagnostics and Clinical Laboratory Director for Quadrant Laboratories, said, "In both the pediatric and adolescent populations, we found it was much easier to collect saliva versus blood. Additionally, saliva serves as an excellent source of the genetic material essential for conducting an accurate test to identify Fragile X Syndrome, as well as other Fragile X-related disorders such as Fragile X-associated Tremor/Ataxia Syndrome (FXTAS) and Fragile X-associated Primary Ovarian Insufficiency (FXPOI), as well as carrier screening."
Fragile X Syndrome is one of the most common genetic causes of intellectual disability. This genetic condition is caused by changes in the FMR1 gene, which is responsible for creating a protein crucial in brain development. Following the discovery of the association between the FMR1 gene and FXS, gene-specific testing has made identifying the condition significantly more accurate.
Bryan Greene, CEO of Quadrant Laboratories, said, "Testing for Fragile X syndrome identifies over 99% of individuals with the syndrome and identifies carriers of the FMR1 premutation."
Based on recommendations from American Academy of Pediatrics, any child who presents with a developmental delay, a diagnosis of autism without a specific etiology, borderline intellectual abilities, or has a family history of FXS should undergo genetic testing for Fragile X Syndrome.
For physicians who are interested in ordering a test, they can visit quadrantlaboratories.com/fxs-asd-id-panel/.
About Quadrant Laboratories Quadrant Laboratories is a wholly owned subsidiary of Quadrant Biosciences, a life sciences company dedicated to improving the lives of children and families by delivering innovative diagnostic, therapeutic, and virtual care solutions for global health priorities. Through the development of novel diagnostics, Quadrant Laboratories provides solutions that accelerate the diagnostic timeline and deliver scientifically sound results earlier allowing individuals to make informed healthcare decisions.
For PR inquiries, email PR@QuadrantBiosciences.com.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/new-york-state-laboratory-approved-for-genetic-testing-using-saliva-301959200.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/new-york-state-laboratory-approved-for-genetic-testing-using-saliva-301959200.html
SOURCE Quadrant Laboratories