New York
Looking for an IT job? From data engineer to information security, check out the BioSpace list of 10 companies hiring life sciences professionals like you.
Looking for a research and development job? Check out the BioSpace list of 12 companies hiring life sciences professionals like you.
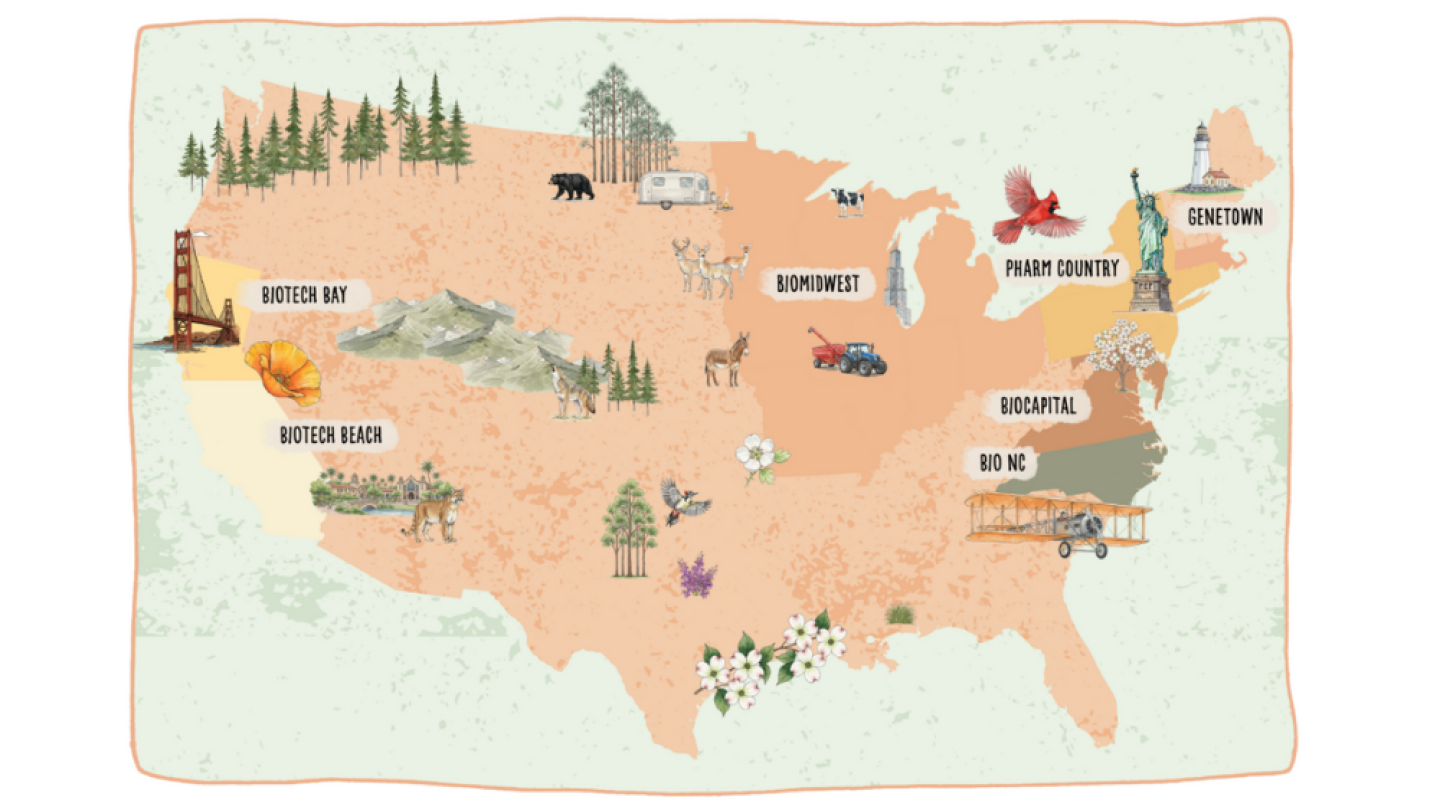
BioSpace has revealed its new 2026 Hotbed Maps, showcasing seven regional life sciences hot spots.
In 2025, made or projected biopharma workforce cuts affected about 42,700 employees, according to BioSpace tallies. BioSpace takes a deep dive into which companies and locations were impacted and speaks to experts about what to expect ahead—and why.
Looking for a biopharma job in New York? Check out the BioSpace list of eight companies hiring life sciences professionals like you.
Looking for a job in regulatory? Check out the BioSpace list of eight companies hiring life sciences professionals like you.
Looking for a job in oncology? Check out the BioSpace list of nine companies hiring life sciences professionals like you.
This year, Novo Nordisk and Merck announced significant layoffs, with Novo planning to axe about 9,000 employees and Merck projecting it could let go of roughly 6,000. Meanwhile, Bayer, Bristol Myers Squibb, Novartis and Pfizer have also made noteworthy cuts.
New York City has seen increased life sciences employment during the past decade as public funding and key projects like JLABS @ NYC have given the area a boost. A Partnership Fund for New York City executive discusses the city’s strengths and a notable challenge facing businesses.
The biopharma job market failed to turn around in May, but employers were still hiring, especially in Indiana and California, based on BioSpace data. The two states had the most job postings live on BioSpace last month, with Indiana showing a 108% year-over-year increase.
PRESS RELEASES