Northern California
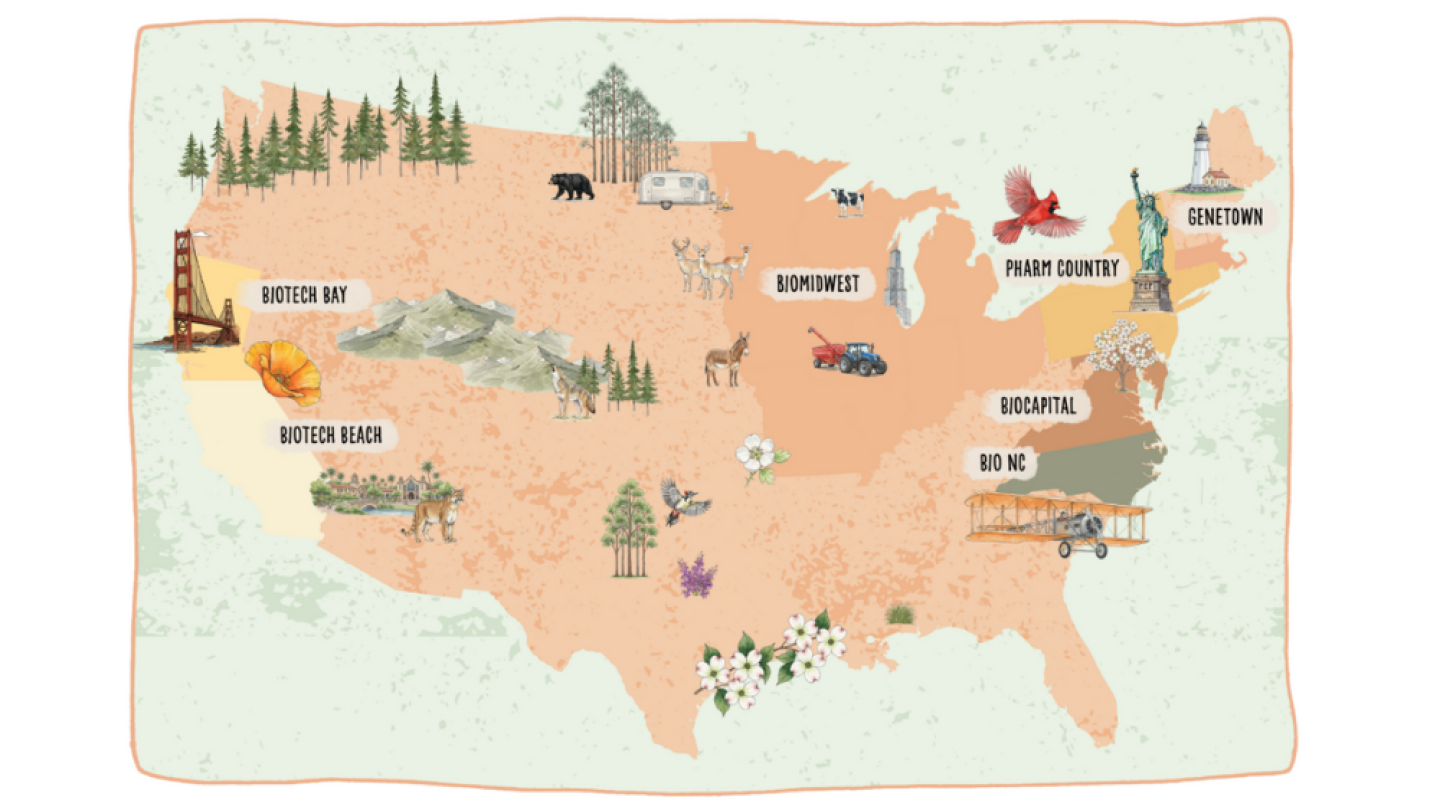
BioSpace has revealed its new 2026 Hotbed Maps, showcasing seven regional life sciences hot spots.
BioSpace has revealed its 2025 Hotbed Maps, showcasing nine regional hot spots for life sciences activity.
Tenaya’s share slump following the TN-201 data drop could be due to its “significantly lower” level of RNA expression in the Phase Ib/II trial than in preclinical models, according to William Blair analysts.
The discontinuation of STRIDES is a rare stumble for the next-generation obesity field and comes just weeks after Amgen announced underwhelming mid-stage data for MariTide.
Astellas Gene Therapies is closing its San Francisco biomanufacturing facility, shifting gene therapy manufacturing to North Carolina, cutting at least 17 employees and affecting dozens more.
Clinical trial concerns and a negative advisory committee vote ultimately sunk the treatment.
Sangamo Therapeutics announced Tuesday it secured an exclusive licensing agreement with Roche’s Genentech, which is paying $50 million in near-term upfront fees and milestone payments to develop novel genomic medicines for neurodegenerative diseases.
Ultracompact CRISPR systems, which are in some cases one-third the size of Cas9, are being designed to be more specific and enable in vivo gene editing in difficult to reach tissues.
While many describe California as having a tough life sciences market, there’s some optimism that employment opportunities will improve soon, according to California Life Sciences President and CEO Mike Guerra.
Sangamo and Pfizer’s hemophilia A gene therapy candidate scored a Phase III victory last week. However, with the genomic medicine company soon to run out of cash, Sangamo’s short-term prospects look bleak but not unsalvageable, analysts say.
PRESS RELEASES