NorthShore University HealthSystem (NorthShore) has joined researchers at Johns Hopkins Medicine to test the effectiveness of convalescent blood plasma outpatient treatments
|
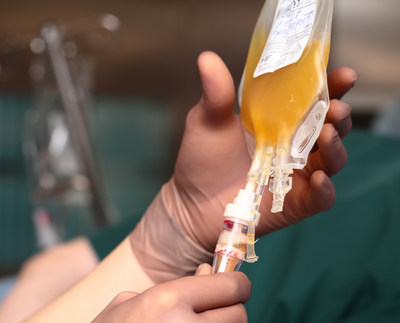
EVANSTON, Ill., Dec. 8, 2020 /PRNewswire/ -- NorthShore University HealthSystem (NorthShore) has joined researchers at Johns Hopkins Medicine to test the effectiveness of convalescent blood plasma outpatient treatments. NorthShore is one of 30 sites throughout the country participating in these randomized, double-blind, controlled, phase II trials and is the only site in the state of Illinois. The research is being funded by the U.S. Department of Defense and the National Institutes of Health. Currently, only hospitalized patients have access to convalescent blood plasma for treatment of COVID-19. "We are excited to be a part of this important research," said Giselle Mosnaim, MD, who is leading the studies at NorthShore. "These studies will give us the clinical data necessary to determine if convalescent blood plasma therapy can prevent infection and help patients recover more quickly in the early stages of the disease." The research will also provide scientists with a good benchmark on how the immune system protects people from COVID-19 -- a key piece of evidence when it comes to evaluating vaccines. Convalescent blood plasma therapy involves transfusing a portion of blood called plasma from people who have recovered from the virus and demonstrate a strong immune response. When separated from red and white blood cells and platelets in the blood, plasma is the yellow-tinged liquid that includes proteins called antibodies. These antibodies glom on to foreign substances such as viruses and either mark them for destruction by the immune system or disrupt a virus' ability to multiply and grow. For both trials, a computer will assign participants by chance to get either plasma with COVID antibodies or plasma without COVID antibodies. The infection prevention trial aims to recruit 500 participants and is designed to test whether giving blood convalescent plasma will prevent illness or lessen the severity of illness in people who are at risk of developing COVID-19 after getting exposed to someone who is infected. Participants being asked to join this study must be 18 years of age or older and have had a close contact exposure to a person with COVID-19 within the past 4 days, and not have active COVID-19 infection. Per the CDC guidelines, a close contact exposure is defined as anyone who was within 6 feet of an infected person for at least 15 minutes. Participants in the prevention trial must consent to a nasal swab test for COVID-19, a plasma infusion, medical history and physical exam, blood tests and up to seven in-person visits to the study site over 90 days. Separately, the early treatment plasma trial goal is to recruit 600 participants who have early COVID-19 disease, meaning they are within days of their first symptoms but are not sick enough to be hospitalized. Participants must be 18 years of age or older, test positive for COVID-19 and present with onset of symptoms within the past eight days (e.g. cough, shortness of breath, fever, chills, muscle pain, headache, sore throat, new loss of taste or smell, neurological changes or new skin rash). Participants in the early treatment trial must consent to a nasal swab test for COVID-19, a plasma infusion, medical history and physical exam, blood tests, up to five in-person study visits over 90 days and keep a daily symptom log. For more information, call 224-364-7473; email plasmastudy@northshore.org or visit northshore.org/covid-studies. About NorthShore University HealthSystem
SOURCE NorthShore University HealthSystem |