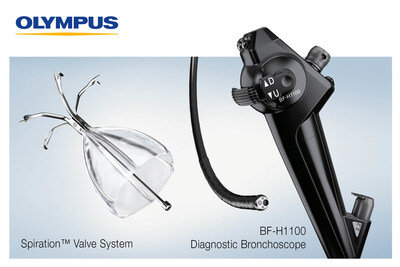
Olympus announced today that it will highlight its algorithm-powered emphysema screening program along with two exciting bronchoscopes compatible with the EVIS X1™ endoscopy system as part of the Olympus Hybrid Bronchoscopy Solution during the annual American Thoracic Society meeting May 17-22 in San Diego.
|
CENTER VALLEY, Pa., May 15, 2024 /PRNewswire/ -- Olympus, a global medical technology company committed to making people's lives healthier, safer and more fulfilling, announced today that it will highlight its algorithm-powered emphysema screening program along with two exciting bronchoscopes compatible with the EVIS X1™ endoscopy system as part of the Olympus Hybrid Bronchoscopy Solution during the annual American Thoracic Society meeting May 17-22 in San Diego. SeleCT™ Screening, an algorithm-driven screening process, reviews existing chest CT scans to help identify patients who would benefit from bronchoscopic lung volume reduction (BLVR). BLVR with endobronchial valves such as the Spiration™ Valve may improve lung function by redirecting air away from hyperinflated portions of the lung to healthier portions.1 Study results show that patients treated with the Spiration Valve System experienced a significant improvement in lung function, respiratory symptoms and quality-of-life scores at 24 months. Now with the addition of SeleCT Screening, an algorithm can help healthcare providers find more appropriate patients, potentially bringing BLVR with the Spiration Valve to even more patients suffering with emphysema. Chronic Obstructive Pulmonary Disease (COPD), which includes emphysema, is a commonly undiagnosed lung disease with studies showing that as many as 80% of adults with COPD worldwide are undiagnosed. Early detection of COPD is important because properly managing the disease has shown to reduce the risk of future exacerbations and hospitalizations and to alleviate symptoms.2 Potential adverse events which may be associated with the use of the Spiration Valve System may include, but are not limited to, pneumothorax, worsening of COPD symptoms, pneumonia, and dyspnea. A full list of prescriptive information and additional information on indications, contraindications, warnings, precautions and potential complications is available here. Compatible with the EVIS X1™ endoscopy system, the BF-H1100 diagnostic bronchoscope and the BF-1TH1100 therapeutic bronchoscope each feature a compact outer diameter and large working channel, as well as high-definition imaging. Education will be a primary focus at this year's annual ATS meeting with Olympus hosting a series of workshops and peer-to-peer hands-on experiences on various topics, including:
Spiration Valve is authorized by federal law as a Humanitarian Use Device for use in the control of prolonged air leaks of the lung or significant air leaks that are likely to become prolonged air leaks, following lobectomy, segmentectomy, or Lung Volume Reduction Surgery (LVRS). The effectiveness of this device for this use has not been demonstrated. Potential complications which may be associated with bronchoscopy and/or the Spiration Valve System may include, but are not limited to, pneumothorax, atelectasis, pneumonia, shortness of breath, and in rare cases, death. A full list of prescriptive information and additional information on indications, contraindications, warnings, precautions and potential complications is available here. For more information about the pulmonology portfolio, visit the Olympus booth, #2543, during ATS or visit the pulmonology product page for more detail. About Olympus At Olympus, we are committed to our purpose of making people's lives healthier, safer and more fulfilling. As a global medical technology company, we partner with healthcare professionals to provide solutions and services for early detection, diagnosis and minimally invasive treatment, aiming to improve patient outcomes by elevating the standard of care in targeted disease states. For more than 100 years, Olympus has pursued a goal of contributing to society by producing products designed with the purpose of delivering optimal outcomes for its customers around the world. Olympus Corporation of the Americas, a wholly owned subsidiary of Olympus Corporation, is headquartered in Center Valley, Pennsylvania, USA, and employs more than 4,500 employees throughout locations in North and South America. For more information, visit olympusamerica.com. 1 Criner GJ, Delage A, Voelker K, et al. Improving Lung Function in Severe Heterogenous Emphysema with the Spiration Valve System (EMPROVE). A Multicenter, Open-Label Randomized Controlled Clinical Trial. Am J Respir Crit Care Med. 2019;200(11):1354-1362. doi:10.1164/rccm.201902-0383OC
SOURCE Olympus Corporation of the Americas |