Bringing new drugs to the market costs billions of dollars. It could not be done without investments by both the NIH and biopharma companies.
Pictured: Illustration of scientists working on biomedical research/iStock, Olga Kurbatova
There is a stark divide in public depictions of pharmaceutical innovation. The biopharma industry proudly advertises its accomplishments in launching new drugs for cancer, neurodegenerative disease, diabetes, weight loss and more without acknowledging the billions of dollars of government funding for basic and applied biomedical science that enabled these advances. Industry critics, meanwhile, argue that this government funding is responsible for pharmaceutical innovation, going as far as to propose that patients “pay twice” for medicines, once in the form of taxes that underwrite government research and again in purchasing these products.
Two recent papers published by my colleagues and me in the Journal of the American Medical Association’s Health Forum suggest that neither view is accurate. Our research illustrates the essential complementarity of government and industry spending on new medicines.
True Partners in Drug Development
There is no disagreement on the process leading to new drugs. Contemporary pharmaceutical innovation is based on a deep foundation of basic research that describes the biology of both health and disease as well as mechanisms and targets for medicines that may preserve or restore health, or at least reduce the morbidity and mortality associated with disease.
This leads to applied research to identify candidate drug molecules and understand their activity in laboratory and animal models. Promising compounds then enter clinical development, which explicitly focuses on the human trials necessary to establish the efficacy and safety of these products, establish manufacturing capacity and satisfy the requirements for marketing approval by the FDA or other regulatory agencies.
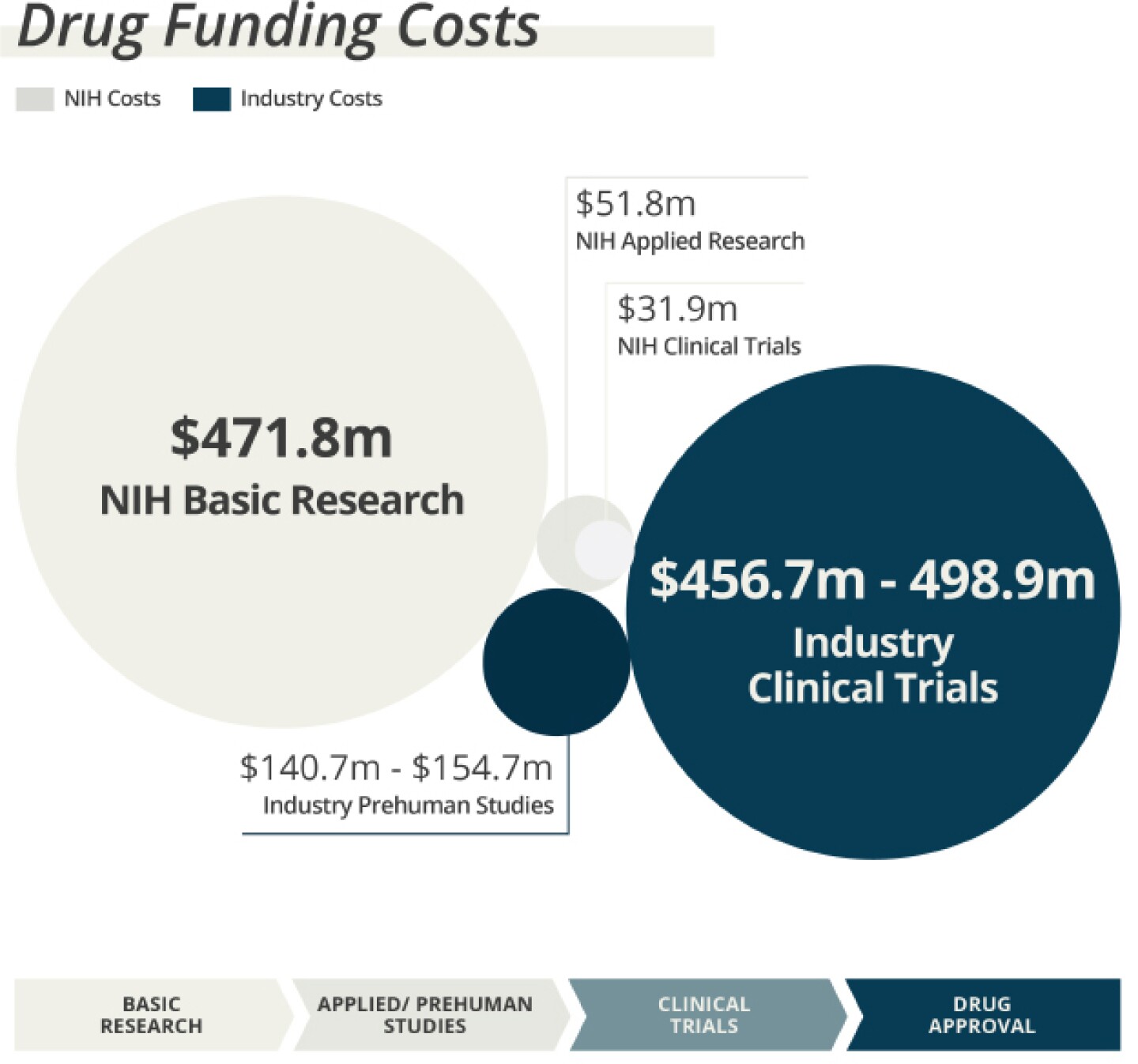
My colleagues and I at the Center for Integration of Science and Industry at Bentley University have undertaken a series of studies, supported by the Institute for New Economic Thinking, National Biomedical Research Foundation and National Pharmaceutical Council, to characterize the U.S. government’s investment in this process through the National Institutes of Health (NIH).
In our first two studies, published in 2018 and 2020, we identified $187 billion in NIH funding for research related to the 356 drugs approved between 2010 and 2019. Of this amount, ~83% represents basic research related to the drug target and ~17% applied research related to the drugs themselves. This April, we published a third study demonstrating that the value of the NIH investment in new drug approvals was comparable to reported levels of investment by industry.
A subsequent study, published July 14, focused explicitly on NIH funding for clinical development. Here we demonstrated that only 3.3% of all NIH funding related to new drug approvals pertains to clinical trials. That means that when it comes to this phase of drug development, the NIH invests only ~10% of the spending from the biopharma industry. We further showed that >90% of the NIH funding contributing to clinical development was in the form of grants supporting clinical consortia or centers, or translational science methods, capacity or training, rather than the clinical trials themselves.
Taken together, these studies demonstrate the essential complementary nature of government and industry investments in pharmaceutical innovation. Industry has limited incentive to invest in basic science, which typically produces results that may not be patentable, are widely disseminated in scientific journals and provide uncertain returns to the company making the investment. By funding this research, the government reduces the industry’s total investment cost in new drug approvals by approximately half and creates economic efficiencies through spillover effects in which multiple companies are able to use the same scientific knowledge to develop different products.
In contrast, industry has multiple incentives to focus on clinical development, which not only allows commercialization of new products but also generates patents, know-how and trade secrets that contribute to market exclusivity and industry’s ability to set prices and maximize profits.
Ensuring Equitable Returns for All Parties
It is increasingly recognized that government plays an essential role as an early investor in technological innovation, making investments in research that is too uncertain or products that are too distant for industry to justify to their shareholders.
These investments reduce the risk of subsequent industry investments necessary to develop and commercialize practical applications of this research. As such, economists argue that taxpayers could expect returns on their investments in this process commensurate with those of pharmaceutical companies or their shareholders and the relative risks of these contributions. These may include both economic returns to public-sector organizations and social returns in the form of improved health or wellness, lower healthcare costs, new scientific insights or jobs.
Any attempt to deliver such public returns is undermined, however, by the disparate descriptions of the investments leading to new drugs promoted by the pharmaceutical industry and its critics—descriptions that belie the essential interdependence of public and private sector investments in this arena. Pharmaceutical innovation is not advanced by rhetoric that dismisses the contributions of either sector nor by partisan pressures that advocate for one sector at the expense of the other. Rather, effective innovation may be advanced through greater transparency concerning the investment and activities necessary for bringing drugs to market.
Only by acknowledging the contributions of both sectors will society be able to address unmet medical needs, identify potential economic and operational synergies that could make innovation more efficient and assure equitable returns on both public and private investments in pharmaceutical innovation.
Fred Ledley, M.D., is the director of the Center for Integration of Science and Industry at Bentley University in Massachusetts. He has served previously on the faculties of the Howard Hughes Medical Institute and Baylor College of Medicine, as a Founder and VP of R&D at GeneMedicine Inc. and as president and CEO of Variagenics Inc.