Ortho Development Corporation, a designer and manufacturer of orthopedic implants and instruments for hip and knee joint replacement surgery, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of the Trivicta™ Hip System.
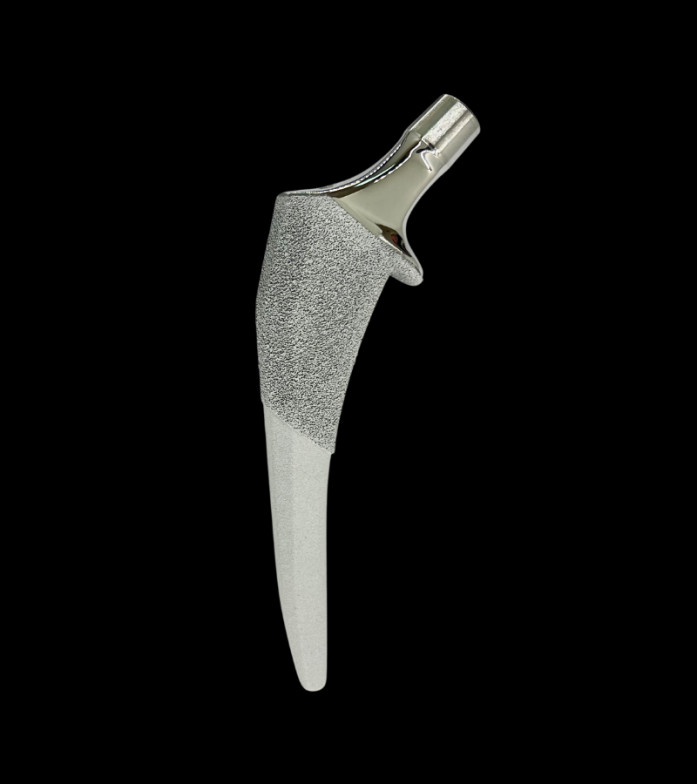
DRAPER, UT / ACCESSWIRE / March 14, 2024 / Ortho Development Corporation, a designer and manufacturer of orthopedic implants and instruments for hip and knee joint replacement surgery, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of the Trivicta™ Hip System. The triple-taper femoral stem facilitates an optimal fit within the canal for a diverse range of patient anatomies.
"Trivicta expands our extensive primary hip portfolio and incorporates our philosophy of evolutionary innovation. The system is designed to be versatile enough to address the vast needs of patients and surgeons, accommodating both muscle-sparing and traditional surgical approaches," said Brent Bartholomew, President & CEO of Ortho Development.
Designed for initial and long-term stability, Trivicta boasts a triple-tapered geometry in three separate planes and incorporates two coatings. Sintered beads provide initial stability and allow ingrowth for long-term stability, while Hydroxyapatite promotes biological fixation. Additionally, extraction and compaction broaching are integrated in one broach to preserve bone while ensuring optimal fit.
Dr. Marc Hungerford, an orthopedic surgeon at Mercy Medical Center in Baltimore, MD, emphasized the benefits of the system: "The triple-taper stem geometry of Trivicta should result in a precise fit for most femoral morphologies. The unique advantage of this design is the ability to use extended offset to deliver direct lateralization independent of leg length."
Trivicta will be available in the United States in the second quarter on a limited basis, with a full commercial release to follow.
About Ortho Development Corporation
Ortho Development Corporation designs, manufactures, and distributes orthopedic implants and related surgical instruments. The company's principal product focus is total knee and hip joint replacement. To learn more visit: www.odev.com.
Contact: Phone: +1 801-553-9991 | Email: marketing@odev.com
Contact Information:
Dawn Boyd
Director of Marketing and Communications
dawn.boyd@orthodevelopment.com
512-508-5665
SOURCE: Ortho Development
View the original press release on newswire.com.