Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced statistically significant 12-month results from a large, multicenter randomized controlled trial (RCT) that further validate the superiority of DTM™ Spinal Cord Stimulation (SCS) in providing back pain relief, compared with conventional SCS therapy, using the Medtronic Intellis
|
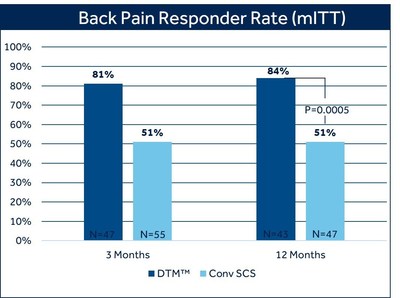
DUBLIN, Oct. 20, 2020 /PRNewswire/ -- Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced statistically significant 12-month results from a large, multicenter randomized controlled trial (RCT) that further validate the superiority of DTM™ Spinal Cord Stimulation (SCS) in providing back pain relief, compared with conventional SCS therapy, using the Medtronic Intellis™ platform. At 12 months, 84% of patients with chronic back pain treated with DTM SCS reported at least 50% pain relief, compared to 51% of patients treated with conventional SCS (p=0.0005).1 There was also a difference in the proportion of patients who reported profound back pain relief (>80% reduction in VAS score) favoring DTM SCS (69%) compared with conventional SCS (35.1%).1 The study met its primary endpoint at three months, and in pre-specified secondary analysis showed the superiority of DTM SCS compared to conventional SCS and has sustained these results at 12 months. Pain relief was measured by the Visual Analog Scale (VAS), a widely used and accepted measure for pain intensity that records patient-reported pain levels on a scale of 0-10. Fifty-percent pain relief, as measured by VAS, is a recognized industry standard to define therapy success. The majority of DTM SCS patients in this study exceeded this threshold, with seven out of ten experiencing profound back pain relief at 12 months. Patients treated with DTM SCS also reported an average VAS score reduction of 75% in back pain, compared with 50% treated with conventional SCS. Average VAS scores for patients treated with DTM SCS at 12 months were 1.74 for back pain and 1.45 for leg pain.1 The term remitter has previously been used to classify patients with a pain score of 2.5 or less. As a group, patients in the DTM SCS group clearly fell below this level with a mean VAS score of 1.74 for back pain and 1.4 for leg pain. "DTM SCS has the potential to improve outcomes for patients with chronic back pain," said Michael Fishman, M.D., MBA, anesthesiologist and Interventional Pain Medicine specialist at the Center for Interventional Pain & Spine. "More than two-thirds of the patients in this RCT achieved profound pain relief of at least 80% with DTM SCS therapy. These outcomes demonstrate that DTM SCS can provide more patients profound pain relief compared with conventional SCS at 12 months, which physicians should consider when selecting a neuromodulation therapy for their patients." "DTM SCS is based on a novel understanding of how neurons and glial cells contribute to chronic pain," said Charlie Covert, vice president and general manager of the Pain Therapies business, part of the Restorative Therapies Group at Medtronic. "The 12-month data reported demonstrate the value of Medtronic's continued focus on pursuing science-based approaches to improving human health and underscore our ability to integrate existing technologies with novel therapies like DTM SCS to improve the outcomes of people suffering from chronic pain." Go to Medtronic.com/DTMwebinar to register for a webinar on Tues. Oct. 20 at 12 p.m. CDT to learn more about the clinical trial results. About the Trial About DTM SCS Therapy About the Intellis SCS Platform About Medtronic Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results. References
SOURCE Medtronic plc |
||||||||||
Company Codes: NYSE:MDT |
||||||||||