MONTVALE, N.J., Aug. 6, 2024 /CNW/ -- PENTAX Medical America Inc., a division of the HOYA Group, is proud to announce two significant updates to its C2 CryoBalloon product line. These advancements are designed to enhance patient care and expand treatment options for gastroenterologists.
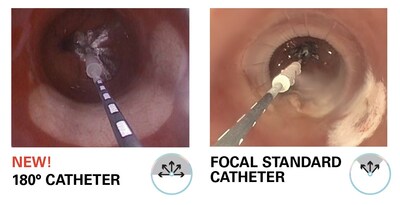
1. 180° C2 CryoBalloon™ Catheter for Barrett's Esophagus:
- Increased Treatment Area and Efficiency: The 180° C2 CryoBalloon Catheter is the first cryogenic product specifically designed to treat large areas of Barrett's esophagus. The unique 180° design allows gastroenterologists to cover larger areas quickly, reducing the overall procedure time. This establishes the C2 CryoBalloon 180° as an ablative therapy that treats large tissue areas, minimizes patient discomfort, and demonstrates 90% disease regression at the first surveillance visit.
2. New Clinical Indication for Gastric Antral Vascular Ectasia (GAVE):
- Less Painful Treatment Option: The C2 CryoBalloon solution now offers a novel treatment for GAVE. This minimally invasive cryogenic technology proves to be a less painful alternative to existing available treatment. C2 CryoBalloon System targets affected areas with quick precise cryotherapy, minimizing the need for frequent packed red blood cell transfusions at 6 months when compared to existing treatment modalities.
- FDA Clearance: PENTAX Medical has obtained FDA clearance for the use of the C2 CryoBalloon system in treating GAVE. This clearance confirms the system's safety and efficacy, offering gastroenterologists a new tool to enhance patient care.
"The introduction of the 180° C2 CryoBalloon Catheter and the new indication for GAVE treatment represent significant advancements in patient care." This was emphasized by David Hedrick, Senior Director of the C2 CryoBalloon Franchise. "These innovations not only provide more effective and less painful treatment options but also greatly expand the application of the C2 CryoBalloon technology. By offering faster and safer procedures, we are enhancing the quality of life for patients and giving gastroenterologists powerful new tools to address complex conditions."
For more information, please visit www.pentaxmedical.com/us.
About PENTAX Medical
PENTAX Medical is a division of HOYA Group. The company's mission is to improve the standard of patient care and quality of healthcare delivery by providing the best endoscopic products and services with a focus on QUALITY, CLINICALLY RELEVANT INNOVATION, and SIMPLICITY.
PENTAX Medical strives to align with the healthcare community's Triple Aim goals through transparent partnerships with its customers and by providing the highest quality solutions to help them reach their goals, including enabling customers to improve patient outcomes by offering evidence-based solutions across the continuum of care; ensuring value by supporting the customers to improve their efficiency and minimize their healthcare costs; and enriching patient and provider's experience by empowering every member of the care team to achieve optimal outcomes through products, education, and support.
Focused on the outcome instead of technological features, PENTAX Medical listens to the healthcare community and their patients, understands their daily obstacles and helps improve endoscopy with smart innovations.
For more information: http://www.pentaxmedical.com
About HOYA
Founded in 1941 in Tokyo, Japan, HOYA Corporation is a global technology and med-tech company and a leading supplier of innovative high-tech and medical products. HOYA is active in the fields of healthcare and information technology, providing eyeglasses, medical endoscopes, intraocular lenses, and optical lenses, as well as key components for semiconductor devices, LCD panels, and hard disk drives. With over 150 offices and subsidiaries worldwide, HOYA currently employs a multinational workforce of 37,000 people. For more information, please visit: http://www.hoya.com.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/pentax-medical-introduces-new-updates-to-c2-cryoballoon-product-line-302213803.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/pentax-medical-introduces-new-updates-to-c2-cryoballoon-product-line-302213803.html
SOURCE PENTAX Medical