Peregrine Ventures, a leading investment firm dedicated to supporting life changing healthcare opportunities, today realized the USD 500 million exit from CartiHeal (USD 350 million down payment plus an additional USD 150 million milestone).
|
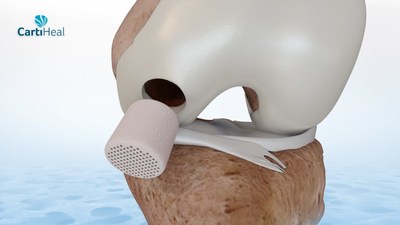
CartiHeal's Acquisition by Bioventus Will Proceed as Previously Announced Following This Milestone Achievement OR YEHUDA, Israel, April 6, 2022 /PRNewswire/ -- Peregrine Ventures, a leading investment firm dedicated to supporting life changing healthcare opportunities, today realized the USD 500 million exit from CartiHeal (USD 350 million down payment plus an additional USD 150 million milestone). This exit follows the portfolio company's Food and Drug Administration (FDA) approval of the Agili-C™ implant for the treatment of cartilage and osteochondral defects. Having achieved this milestone, the medical device company's previously announced acquisition by Bioventus, in one of the largest medical acquisition deals in Israel in the last 12 months, will continue as planned. "Upon first hearing of CartiHeal's groundbreaking technology, we knew of the company's immense potential. In fact, we were CartiHeal's first investors," said Peregrine's Co-founder and Managing General Partner, Boaz Lifschitz. "Over USD 7 billion has been spent worldwide to deliver a cartilage treatment technology which has yielded insufficient treatment options, until now. It has been thrilling to work with Nir Altschuler and his team to help bring to market a cartilage treatment that will improve the quality of life of millions of people around the world." Over a decade ago, CartiHeal began its journey as part of Peregrine's award-winning “Incentive” Technology Incubator. Here, Peregrine assisted the early-stage company with its business and product development offering creative business solutions and funding connections. Over the course of its partnership with CartiHeal, Peregrine, offered guidance on business operations and assisted with the company's staffing. Founded by Nir Altschuler in 2009, CartiHeal produces a much-needed, biodegradable treatment for cartilage and osteochondral defects in arthritic and non-arthritic knee-joints. Following a robust clinical study in which 251 patients were enrolled in 26 sites in the US, Europe, and Israel, where the superiority of the Agili-C™ implant over the current Surgical Standard of Care (SSOC), microfracture and debridement, was confirmed for the treatment of knee joint surface lesions, chondral and osteochondral defects. Previously, the implant was granted Breakthrough Device Designation by the FDA in 2020. "It is thrilling to receive FDA approval which will allow us to provide quality care for millions of patients that would have otherwise been faced with no viable treatment for degenerative knee cartilage," said Mr. Altschuler, CartiHeal's Founder & CEO. "Through many years of partnership with Peregrine, we achieved a scientific breakthrough. As a former manager at the investment firm, it has been incredible to experience first-hand the commitment Peregrine makes in its portfolio companies, from capital and connections to business development and guidance." This successful exit follows Peregrine's USD 300 million exit from Cardiovalve, a pioneering transcatheter mitral and tricuspid valve replacement company earlier this year, bringing total proceeds of the deal to USD 1 billion. ABOUT PEREGRINE VENTURES ABOUT CARTIHEAL PEREGRINE VENTURES RUBENSTEIN PUBLIC RELATIONS
SOURCE Peregrine Ventures |