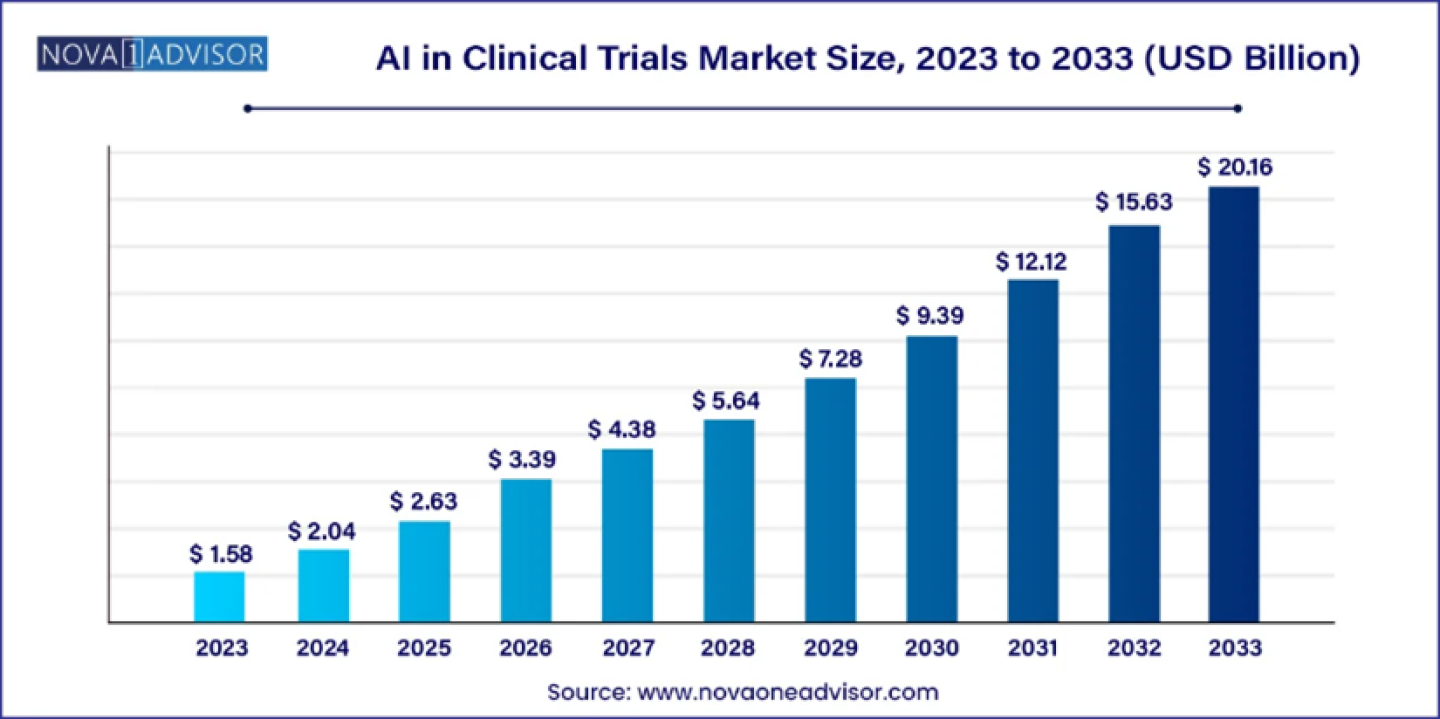
According to Nova One Advisor, the global AI in clinical trials market size was USD 1.58 billion in 2023, calculated at USD 2.04 billion in 2024 and is expected to reach around USD 20.16 billion by 2033, expanding at a CAGR of 29% from 2024 to 2033.
The increasing urgent requirement to cut expenses and speed up medication development, increasing demand for tailored medicine, increasing trend towards personalized medicine, and increasing recognition of AI in clinical trials by regulatory agencies are expected to drive the market growth.
Full Report is Ready | Ask here for Sample Copy@ https://www.novaoneadvisor.com/report/sample/8875
AI in Clinical Trials Market Key Takeaways
AI in clinical trials market can revolutionize the entire drug development procedure. AI technologies can cheaply and quickly sort through huge amounts of data to find individuals who cut down on recruitment time and expenses and address the requirements. AI reveals hidden patterns, boosts insights from large datasets, and streamlines data analysis. Artificial intelligence can help researchers create more productive and successful studies evaluate past data and forecast future results. According to health and genetic characteristics, AI in clinical trials can customize a patient’s course of care.
In addition, the impending patent expiry of blockbuster drugs, the rising adoption of cloud-based services and applications, and the increasing need to reduce the time involved and curb clinical trial costs are expected to drive the growth of the AI in clinical trials market. Furthermore, the growing usage of AI-based platforms, rising technological developments, and increasing research and development activities are further accelerating the market growth during the forecast period.
Immediate Delivery is Available | Get Full Report Access@ https://www.novaoneadvisor.com/report/checkout/8875
Use Cases of AI in Clinical Trials Market
Artificial Intelligence offers various valuable use cases in clinical trials, redefining the way research and development processes are conducted in the healthcare industry. The use of AI in clinical trials can revolutionize the entire drug development process, enabling more efficient data management, improved decision-making, and overall success of the clinical trial value chain.
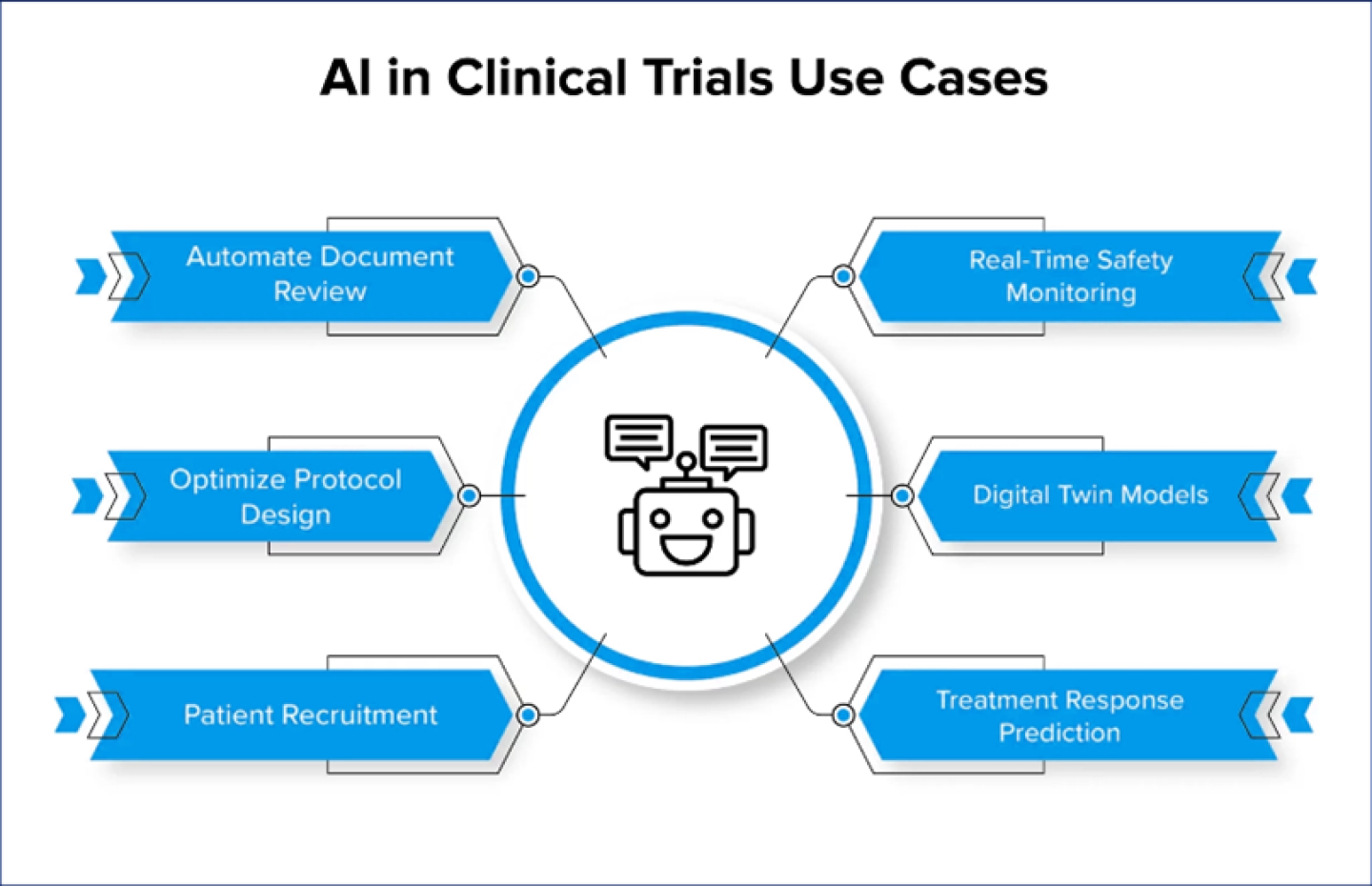
Here are some of the most prominent use cases of AI in clinical trials:
Automate Document Review
· Artificial intelligence in clinical trials helps review and analyze regulatory documents, such as Investigational New Drug (IND) applications. It helps identify errors, inconsistencies, or missing information, ensuring compliance with regulatory standards and accelerating the submission process.
Optimize Protocol Design
· The use of AI in clinical trials starts from the initial stages, where it transforms the way study protocols are designed. By analyzing historical data, the technology suggests protocol improvements, defines endpoints, and recommends patient recruitment criteria, leading to more efficient and scientifically robust trials.
Patient Recruitment
· Artificial intelligence in clinical trials analyzes patient data, electronic health records (EHR), and medical literature to match eligible patients with specific trial criteria. While selecting the patients for clinical trials, AI assesses various factors, including geographical locations, patient demographics, and site performance history. This speeds up patient recruitment and ensures a more precise selection process.
Real-Time Safety Monitoring
· AI continuously monitors clinical trial data for safety signals and adverse events. By analyzing patient data in real-time, AI plays a crucial role in improving clinical trials with machine learning by promptly identifying potential safety concerns. This enables immediate actions to protect patient safety and ensure regulatory compliance.
Digital Twin Models
· One of the most groundbreaking applications of AI in clinical trials is the idea of digital twins. Artificial intelligence in clinical trials can create virtual replicas of patients based on their genetic, medical history, and ongoing health data. These virtual replicas serve as dynamic models that simulate and predict outcomes, ushering in a new age where healthcare is truly safe, effective, and individualized.
Treatment Response Prediction
· Through the use of AI and machine learning in clinical trials predictive models are developed. These models are based on patient characteristics and biomarkers, it helps researchers assess how a particular patient responds to various interventions, optimizing treatment efficiency and reducing risks. This approach can potentially transform personalized medicine, detecting potential issues at an early stage and tailoring therapies to each patient’s unique condition.
Future of Artificial Intelligence in Clinical Trials
The future of artificial intelligence in clinical research is promising as the technology is seemingly advancing at breakneck speed, revolutionizing every phase of the clinical trial value chain.
AI plays an increasingly integral role in accelerating drug discovery and development, from optimizing trial protocols and patient recruitment to enhancing data analysis and safety monitoring. With AI’s capacity to drive precision medicine, identify novel therapies, and simulate trial strategies, it promises faster time to market, reduced costs, and more effective, personalized treatments.
As the technology continues to evolve, it will most likely contribute to more efficient, ethical, and successful clinical trials, benefiting patients and the healthcare industry as a whole.
While there are still safety and efficacy concerns with the applications of AI in clinical trials, the hope is that in the future, AI will take on more responsibilities in the drug development process to guarantee speed, accuracy, and efficiency.
People within the industry should use it as a valuable tool while maintaining a balance between innovation and patient safety to ensure the ethical and responsible use of AI for the benefit of all.
The Role of Artificial Intelligence in Drug Discovery of the Future
When looking into the future of clinical trials, AI is unquestionably a multifaceted technology shaping the drug discovery process. The ideology behind using AI-driven tools in clinical trials is establishing a systemic channel to evaluate vast amounts of information generated during drug research with higher accuracy.
To date, researchers are using AI tools to identify drug molecules and understand disease patterns in patients. However, when intelligence is combined with machine learning, the technology will help researchers analyse large amounts of data to modify therapeutic drugs for positive outcomes. The application of an AI algorithm automates various parts of the drug discovery process, resulting in fast, accurate, and efficient clinical trials for drug approval.
By using artificial algorithms in clinical trials, researchers are able to collect invaluable information about drug efficacy and reduce human errors by automation information analysis tasks. The incorporation of AI-powered information analysis tools will serve as a groundbreaking technology to initiate virtual clinical trials in the future to shorten the time required for drug development and reduce dependency on physical volunteers.
Buy Now Full Report: https://www.novaoneadvisor.com/report/checkout/8875
AI in Clinical Trials Market Segment Insights
By offering type
The services segment held the largest market share in 2023. Services segments offer customized solutions that fit into the workflows of current clinical trials. AI applications are made to address the unique goals and requirements of various healthcare institutions and clinical trials by customizing. Service providers are essential when it comes to negotiating the regulatory environment around clinical trials. By pertinent business norms and healthcare laws, they guarantee that AI applications abide by and give stakeholders reduced risk and peace of mind.
The infectious disease segment dominated the AI in clinical trials market in 2023. The segment is expected to continue the expansion during the estimated period. Complex data sets, such as clinical trial outcomes, epidemiological data, and genetic information are continuously included in infectious diseases. The huge amounts of heterogeneous data may be analyzed and processed by artificial intelligence effectively and enable researchers to make defensible conclusions and obtain new insights. Artificial intelligence algorithm helps to monitor infectious diseases in real time, which helps in response planning and outbreak identification. This capacity is necessary for infectious disease segment growth and successful clinical studies.
The deep learning segment held the largest market share in 2023. Massive amounts of diverse and complex data, such as medical imaging, electronic health records, genetic data, and other data related to health trials are outstanding targets for analysis capabilities and deep learning processing. In addition, effective and precise analysis is easily made by deep learning models, particularly the ability to extract pertinent patterns and characteristics and neural networks from unprocessed data automatically. Clinical studies can be more responsive and effective by using adaptive trials to modify necessary parameters, such as treatment regimens or patient enrollment criteria in response to gathered data.
The pharmaceutical segment dominated the AI in clinical trials market in 2023 and the segment is expected to sustain the dominance throughout the forecast period. Pharmaceutical corporations use AI algorithms to examine large amounts of clinical data quickly. This expedites the medication development process by helping to recognize patterns, forecast patient reactions, and optimize trial methods. Artificial intelligence is employed by the pharmaceutical business to detect and alleviate possible hazards linked to clinical studies. Predictive analytics supports proactive risk management and patient safety by estimating the probability of unfavorable outcomes.
North America held the largest share of AI in the clinical trials market in 2023. The region has led in the achievements of artificial intelligence technology and innovation. Various leading startups, academic organizations, and IT companies focus on creating unique AI solutions for various industries, such as healthcare and clinical trials in the region. It has made significant investment and financial commitments to AI research projects and businesses. The venture capital companies, government funding, and private investors have demonstrated a strong desire to guide the application and advancement of AI technology in healthcare, such as clinical trials.
U.S. AI in Clinical Trials Market Trends
The U.S. is the major country in the healthcare industry. In the U.S., AI can be used to improve and monitor adherence during the clinical trial through tracking of medications and smartphone reminders and alerts.
Due to the increasing prevalence of the aging population and chronic diseases, there is an increasing demand for efficient clinical trials in the region. Countries such as India and China, are investing significantly in healthcare innovation and AI technology to reduce the burden of chronic diseases. A large patient pool and lower operational costs make the region an attractive destination for clinical trials.
China AI in Clinical Trials Market Trends
China is the fastest growing country in the healthcare industry. China holds a prominent position in cell therapy trials and gene therapy along with traditional drug categories. The increasing decentralization of clinical trials, focus on innovative therapies, rising adoption of digital technologies such as e-constant, AI-powered platforms, and telemedicine, and data sharing and collaboration are expected to drive the growth of the AI in clinical trials market in China.
AI in Clinical Trials Market Company Insights
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Nova one advisor, Inc. has segmented the AI in Clinical Trials market.
AI in clinical trials market, By Offering
Browse More Insights:
Artificial Intelligence (AI) in Life Sciences Market : The global artificial intelligence (AI) in life sciences market size was exhibited at USD 2.50 billion in 2023 and is projected to hit around USD 15.45 billion by 2033, growing at a CAGR of 19.98% during the forecast period 2024 to 2033.
Personalized Medicine Outsourcing Market : The global personalized medicine outsourcing market size was valued at USD 102.19 billion in 2023 and is anticipated to reach around USD 323.96 billion by 2033, growing at a CAGR of 12.23% from 2024 to 2033.
Personalized Medicine Biomarkers Market : The global personalized medicine biomarkers market size was exhibited at USD 15.22 billion in 2023 and is projected to hit around USD 66.16 billion by 2033, growing at a CAGR of 15.83% during the forecast period of 2024 to 2033.
Biomarkers Market : The global Biomarkers market size was estimated at USD 81.19 billion in 2023 and is projected to hit around USD 284.76 billion by 2033, growing at a CAGR of 13.37% during the forecast period from 2024 to 2033.
Clinical Trial Equipment & Ancillary Solutions Market : The global clinical trial equipment & ancillary solutions market size was exhibited at USD 2.99 billion in 2023 and is projected to hit around USD 7.01 billion by 2033, growing at a CAGR of 8.9% during the forecast period 2024 to 2033.
Clinical Trial Biorepository & Archiving Solutions Market : The global clinical trial biorepository & archiving solutions market size was exhibited at USD 4.70 billion in 2023 and is projected to hit around USD 10.27 billion by 2033, growing at a CAGR of 8.13% during the forecast period 2024 to 2033.
Clinical Trials Market : The global clinical trials market size was estimated at USD 81.90 billion in 2023 and is projected to hit around USD 153.59 billion by 2033, growing at a CAGR of 6.49% during the forecast period from 2024 to 2033.
Clinical Trial Supplies Market : The clinical trial supplies market size was exhibited at USD 3.55 billion in 2023 and is projected to hit around USD 8.49 billion by 2033, growing at a CAGR of 9.11% during the forecast period 2024 to 2033.
Clinical Trial Supply And Logistics Market : The global clinical trial supply and logistics market size was exhibited at USD 3.80 billion in 2023 and is projected to hit around USD 7.98 billion by 2033, growing at a CAGR of 7.7% during the forecast period of 2024 to 2033.
Clinical Trial Management Services Market : The global clinical trial management services market size was valued at USD 31.18 billion in 2023 and is anticipated to reach around USD 69.02 billion by 2033, growing at a CAGR of 8.27% from 2024 to 2033.
Clinical Trials Support Services Market : The global clinical trials support services market size was exhibited at USD 21.80 billion in 2023 and is projected to hit around USD 45.73 billion by 2033, growing at a CAGR of 7.69% during the forecast period 2024 to 2033.
Clinical Trial Imaging Market : The global clinical trial imaging market size was exhibited at USD 1.25 billion in 2023 and is projected to hit around USD 2.61 billion by 2033, growing at a CAGR of 7.65% during the forecast period 2024 to 2033.
Clinical Trial Kits Market: The global clinical trial kits market size was exhibited at USD 3.30 billion in 2023 and is projected to hit around USD 8.18 billion by 2033, growing at a CAGR of 9.5% during the forecast period 2024 to 2033.
Oncology Clinical Trials Market : The global oncology clinical trials market size reached USD 13.19 billion in 2023 and is projected to hit around USD 22.11 billion by 2033, expanding at a CAGR of 5.3% during the forecast period from 2024 to 2033.
Immuno-oncology Clinical Trials Market : The global immuno-oncology clinical trials market size was exhibited at USD 8.30 billion in 2023 and is projected to hit around USD 35.37 billion by 2033, growing at a CAGR of 15.6% during the forecast period 2024 to 2033.
Cell And Gene Therapy Clinical Trials Market : The global cell and gene therapy clinical trials market size reached USD 11.62 billion in 2023 and is projected to hit around USD 47.40 billion by 2033, expanding at a CAGR of 15.09% during the forecast period from 2024 to 2033.
eClinical Solutions Market : The global eclinical solutions market size was valued at USD 9.85 billion in 2023 and is projected to surpass around USD 37.16 billion by 2033, registering a CAGR of 14.2% over the forecast period of 2024 to 2033.
U.S. Clinical Trials Market : The U.S. clinical trials market size was valued at USD 25.81 billion in 2023 and is projected to surpass around USD 41.57 billion by 2033, registering a CAGR of 4.88% over the forecast period of 2024 to 2033.
U.S. Clinical Trial Imaging Market : The U.S. clinical trial imaging market size was valued at USD 409.50 million in 2023 and is projected to surpass around USD 875.93 million by 2033, registering a CAGR of 7.9% over the forecast period of 2024 to 2033.
U.S. Cell And Gene Therapy Clinical Trial Services Market : The U.S. cell and gene therapy clinical trial services market size was valued at USD 7.19 billion in 2023 and is projected to surpass around USD 56.53 billion by 2033, registering a CAGR of 22.9% over the forecast period of 2024 to 2033.
U.S. Pharmaceutical Market : The U.S. pharmaceutical market size was valued at USD 602.19 billion in 2023 and is projected to surpass around USD 1,093.79 billion by 2033, registering a CAGR of 6.15% over the forecast period of 2024 to 2033.
Call: USA: +1 650 460 3308 | IND: +91 87933 22019 |Europe: +44 2080772818
Web: https://www.novaoneadvisor.com/
You can place an order or ask any questions, please feel free to contact at sales@novaoneadvisor.com | +1 650 460 3308
The increasing urgent requirement to cut expenses and speed up medication development, increasing demand for tailored medicine, increasing trend towards personalized medicine, and increasing recognition of AI in clinical trials by regulatory agencies are expected to drive the market growth.
Full Report is Ready | Ask here for Sample Copy@ https://www.novaoneadvisor.com/report/sample/8875
AI in Clinical Trials Market Key Takeaways
- North America held the dominating share of the AI in clinical trials market in 2023.
- By offering, the services segment held the largest market share in 2023.
- By technology, the deep learning segment held the dominating market share in 2023.
- By application, the infectious disease segment dominated the market in 2023.
- By end-user, the pharmaceutical segment held the largest share of the market in 2023; the segment is observed to sustain dominance throughout the forecast period.
AI in clinical trials market can revolutionize the entire drug development procedure. AI technologies can cheaply and quickly sort through huge amounts of data to find individuals who cut down on recruitment time and expenses and address the requirements. AI reveals hidden patterns, boosts insights from large datasets, and streamlines data analysis. Artificial intelligence can help researchers create more productive and successful studies evaluate past data and forecast future results. According to health and genetic characteristics, AI in clinical trials can customize a patient’s course of care.
In addition, the impending patent expiry of blockbuster drugs, the rising adoption of cloud-based services and applications, and the increasing need to reduce the time involved and curb clinical trial costs are expected to drive the growth of the AI in clinical trials market. Furthermore, the growing usage of AI-based platforms, rising technological developments, and increasing research and development activities are further accelerating the market growth during the forecast period.
Immediate Delivery is Available | Get Full Report Access@ https://www.novaoneadvisor.com/report/checkout/8875
Use Cases of AI in Clinical Trials Market
Artificial Intelligence offers various valuable use cases in clinical trials, redefining the way research and development processes are conducted in the healthcare industry. The use of AI in clinical trials can revolutionize the entire drug development process, enabling more efficient data management, improved decision-making, and overall success of the clinical trial value chain.
Here are some of the most prominent use cases of AI in clinical trials:
Automate Document Review
· Artificial intelligence in clinical trials helps review and analyze regulatory documents, such as Investigational New Drug (IND) applications. It helps identify errors, inconsistencies, or missing information, ensuring compliance with regulatory standards and accelerating the submission process.
Optimize Protocol Design
· The use of AI in clinical trials starts from the initial stages, where it transforms the way study protocols are designed. By analyzing historical data, the technology suggests protocol improvements, defines endpoints, and recommends patient recruitment criteria, leading to more efficient and scientifically robust trials.
Patient Recruitment
· Artificial intelligence in clinical trials analyzes patient data, electronic health records (EHR), and medical literature to match eligible patients with specific trial criteria. While selecting the patients for clinical trials, AI assesses various factors, including geographical locations, patient demographics, and site performance history. This speeds up patient recruitment and ensures a more precise selection process.
Real-Time Safety Monitoring
· AI continuously monitors clinical trial data for safety signals and adverse events. By analyzing patient data in real-time, AI plays a crucial role in improving clinical trials with machine learning by promptly identifying potential safety concerns. This enables immediate actions to protect patient safety and ensure regulatory compliance.
Digital Twin Models
· One of the most groundbreaking applications of AI in clinical trials is the idea of digital twins. Artificial intelligence in clinical trials can create virtual replicas of patients based on their genetic, medical history, and ongoing health data. These virtual replicas serve as dynamic models that simulate and predict outcomes, ushering in a new age where healthcare is truly safe, effective, and individualized.
Treatment Response Prediction
· Through the use of AI and machine learning in clinical trials predictive models are developed. These models are based on patient characteristics and biomarkers, it helps researchers assess how a particular patient responds to various interventions, optimizing treatment efficiency and reducing risks. This approach can potentially transform personalized medicine, detecting potential issues at an early stage and tailoring therapies to each patient’s unique condition.
Future of Artificial Intelligence in Clinical Trials
The future of artificial intelligence in clinical research is promising as the technology is seemingly advancing at breakneck speed, revolutionizing every phase of the clinical trial value chain.
AI plays an increasingly integral role in accelerating drug discovery and development, from optimizing trial protocols and patient recruitment to enhancing data analysis and safety monitoring. With AI’s capacity to drive precision medicine, identify novel therapies, and simulate trial strategies, it promises faster time to market, reduced costs, and more effective, personalized treatments.
As the technology continues to evolve, it will most likely contribute to more efficient, ethical, and successful clinical trials, benefiting patients and the healthcare industry as a whole.
While there are still safety and efficacy concerns with the applications of AI in clinical trials, the hope is that in the future, AI will take on more responsibilities in the drug development process to guarantee speed, accuracy, and efficiency.
People within the industry should use it as a valuable tool while maintaining a balance between innovation and patient safety to ensure the ethical and responsible use of AI for the benefit of all.
The Role of Artificial Intelligence in Drug Discovery of the Future
- Pre-discovery/drug discovery stage. This stage involves research on target mechanisms (usually proteins or genes) that cause the disease. Further, the scientists search for molecules or components that could cure the disease or relieve its symptoms. Without AI application, this stage can last up to 4 years.
- Pre-clinical research. At this stage, the scientists define the mode of the components’ action, their efficiency on in vitro and in vivo models, and their potential toxicity. From the drug discovery stage to the end of the pre-clinical research, the work can last up to 7 years.
- Clinical development. This phase consists of three steps, which involve all the regulatory approvals, testing on voluntary individuals, and confirmation of drug efficacy. Even if you use artificial intelligence in drug discovery and development, testing on candidates with diseases is a critical and the most important step.
- Market approval. If the previous phases are successful, the drug is then registered and seeks market approvals from local authorities (such as Marketing Authorization Application in the EU or New Drug Application/ Biologics License Application in the US).
When looking into the future of clinical trials, AI is unquestionably a multifaceted technology shaping the drug discovery process. The ideology behind using AI-driven tools in clinical trials is establishing a systemic channel to evaluate vast amounts of information generated during drug research with higher accuracy.
To date, researchers are using AI tools to identify drug molecules and understand disease patterns in patients. However, when intelligence is combined with machine learning, the technology will help researchers analyse large amounts of data to modify therapeutic drugs for positive outcomes. The application of an AI algorithm automates various parts of the drug discovery process, resulting in fast, accurate, and efficient clinical trials for drug approval.
By using artificial algorithms in clinical trials, researchers are able to collect invaluable information about drug efficacy and reduce human errors by automation information analysis tasks. The incorporation of AI-powered information analysis tools will serve as a groundbreaking technology to initiate virtual clinical trials in the future to shorten the time required for drug development and reduce dependency on physical volunteers.
Buy Now Full Report: https://www.novaoneadvisor.com/report/checkout/8875
AI in Clinical Trials Market Segment Insights
By offering type
The services segment held the largest market share in 2023. Services segments offer customized solutions that fit into the workflows of current clinical trials. AI applications are made to address the unique goals and requirements of various healthcare institutions and clinical trials by customizing. Service providers are essential when it comes to negotiating the regulatory environment around clinical trials. By pertinent business norms and healthcare laws, they guarantee that AI applications abide by and give stakeholders reduced risk and peace of mind.
- For instance, In June 2024, the launch of a new technology platform, One Home for Sites was announced by IQVIA. This platform acts as a single dashboard and single sign-on for the tasks clinical research sites and key systems need to perform across all of the clinical trials it is conducting.
The infectious disease segment dominated the AI in clinical trials market in 2023. The segment is expected to continue the expansion during the estimated period. Complex data sets, such as clinical trial outcomes, epidemiological data, and genetic information are continuously included in infectious diseases. The huge amounts of heterogeneous data may be analyzed and processed by artificial intelligence effectively and enable researchers to make defensible conclusions and obtain new insights. Artificial intelligence algorithm helps to monitor infectious diseases in real time, which helps in response planning and outbreak identification. This capacity is necessary for infectious disease segment growth and successful clinical studies.
- For instance, In June 2024, the United States National Institutes of Health (NIH), such as the National Institutes on Drug Abuse, Infectious Diseases (NIAID) and the National Institute of Allergy announced the launch of two clinical trials testing long-acting human immunodeficiency virus (HIV) pre-exposure prophylaxis through the HIV Prevention Trials Network (HPTN).
The deep learning segment held the largest market share in 2023. Massive amounts of diverse and complex data, such as medical imaging, electronic health records, genetic data, and other data related to health trials are outstanding targets for analysis capabilities and deep learning processing. In addition, effective and precise analysis is easily made by deep learning models, particularly the ability to extract pertinent patterns and characteristics and neural networks from unprocessed data automatically. Clinical studies can be more responsive and effective by using adaptive trials to modify necessary parameters, such as treatment regimens or patient enrollment criteria in response to gathered data.
- For instance, In July 2024, a new research and development project was launched by Fortrea. The aim behind this launch is to craft artificial intelligence-driven clinical trial technologies that make studies safer, speedier, and more efficient.
The pharmaceutical segment dominated the AI in clinical trials market in 2023 and the segment is expected to sustain the dominance throughout the forecast period. Pharmaceutical corporations use AI algorithms to examine large amounts of clinical data quickly. This expedites the medication development process by helping to recognize patterns, forecast patient reactions, and optimize trial methods. Artificial intelligence is employed by the pharmaceutical business to detect and alleviate possible hazards linked to clinical studies. Predictive analytics supports proactive risk management and patient safety by estimating the probability of unfavorable outcomes.
- In June 2023, to enhance the development of Anavex's medication pipeline, Partex Group's exclusive Artificial Intelligence (AI) technology will be utilized in a strategic alliance between Anavex Life Sciences and Partex Group.
North America held the largest share of AI in the clinical trials market in 2023. The region has led in the achievements of artificial intelligence technology and innovation. Various leading startups, academic organizations, and IT companies focus on creating unique AI solutions for various industries, such as healthcare and clinical trials in the region. It has made significant investment and financial commitments to AI research projects and businesses. The venture capital companies, government funding, and private investors have demonstrated a strong desire to guide the application and advancement of AI technology in healthcare, such as clinical trials.
U.S. AI in Clinical Trials Market Trends
The U.S. is the major country in the healthcare industry. In the U.S., AI can be used to improve and monitor adherence during the clinical trial through tracking of medications and smartphone reminders and alerts.
- In August 2024, a specialty tech and AI-enabled CRO, part of Emmes Group, Veridix AI launched its new protocol digitization capabilities. The aim behind this launch was to improve data quality and accelerate clinical trials.
- In February 2024, the US Food and Drug Administration (FDA) focused on how artificial intelligence (AI) can be used to support and improve clinical trials.
- In January 2024, Medable, A US-based clinical trial technology provider introduced AI-powered automation technology that minimizes the amount of time it takes to set up standard clinical trial processes. The aim behind this launch was to streamline manual tasks and save time.
Due to the increasing prevalence of the aging population and chronic diseases, there is an increasing demand for efficient clinical trials in the region. Countries such as India and China, are investing significantly in healthcare innovation and AI technology to reduce the burden of chronic diseases. A large patient pool and lower operational costs make the region an attractive destination for clinical trials.
China AI in Clinical Trials Market Trends
China is the fastest growing country in the healthcare industry. China holds a prominent position in cell therapy trials and gene therapy along with traditional drug categories. The increasing decentralization of clinical trials, focus on innovative therapies, rising adoption of digital technologies such as e-constant, AI-powered platforms, and telemedicine, and data sharing and collaboration are expected to drive the growth of the AI in clinical trials market in China.
- For instance, In June 2023, a generative artificial intelligence (AI)-driven clinical-stage biotechnology company, Insilico Medicine announced that it had completed the first dose in patients in the Phase II clinical trial of INS018_055 and marked the world's first anti-fibrotic small molecule inhibitor designed and discovered using generative AI and initiated phase II clinical trials for further evaluation in China.
AI in Clinical Trials Market Company Insights
- Deep 6 AI is a leading AI-powered precision research platform for life sciences companies and healthcare organizations. Deep 6 reduces months of manual de-risks and data validation and accelerates clinical trials.
- For instance, In January 2023, a leading AI-powered precision research platform, Deep 6 AI designed real-world data services and research algorithms to better prioritize and identify patients for clinical trials within various disease indications with the partnership with Gratitude, an an on-demand, advanced real-world data and advisory services provider.
- AiCure
- Antidote Technologies
- Deep 6 AI
- Mendel.ai
- Phesi
- Saama Technologies
- Signant Health
- Trials.ai
- Innoplexus
- IQVIA
- Median Technologies
- Medidata
- In September 2023, a global pioneer in providing individuals and institutions with reliable intelligence to change the world, Clarivate Plc announced the creation of an Academia & Government Innovation Incubator. This will quicken its approach to fostering creativity, using AI, and launching cutting-edge products for its academic clients and users.
- In July 2023, by advancing the first medication identified and created by generative AI into Phase II clinical trials involving humans, Insilico Medicine has set a new standard in artificial intelligence drug research. The primary program, INS018_055, is a pan-fibrotic inhibitor that may be the first of its kind. Insilico's moonshot medication unequivocally proves the viability of the company's end-to-end AI drug development platform, Pharma. AI.
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Nova one advisor, Inc. has segmented the AI in Clinical Trials market.
AI in clinical trials market, By Offering
- Software
- Phase I
- Phase II
- Phase III
- Services
- Phase I
- Phase II
- Phase III
- Machine Learning
- Deep Learning
- Supervised Learning
- Other Machine Learning Technologies
- Other Technologies
- Oncology
- Nuerological disease and condition
- Cardiovascular diseases
- Metabolic diseases
- Infecstious disease
- Immunology disease
- Other Applications
- Pharmaceutical & biotechnology companies
- Contract research organizations
- Other end users
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)
Browse More Insights:
Artificial Intelligence (AI) in Life Sciences Market : The global artificial intelligence (AI) in life sciences market size was exhibited at USD 2.50 billion in 2023 and is projected to hit around USD 15.45 billion by 2033, growing at a CAGR of 19.98% during the forecast period 2024 to 2033.
Personalized Medicine Outsourcing Market : The global personalized medicine outsourcing market size was valued at USD 102.19 billion in 2023 and is anticipated to reach around USD 323.96 billion by 2033, growing at a CAGR of 12.23% from 2024 to 2033.
Personalized Medicine Biomarkers Market : The global personalized medicine biomarkers market size was exhibited at USD 15.22 billion in 2023 and is projected to hit around USD 66.16 billion by 2033, growing at a CAGR of 15.83% during the forecast period of 2024 to 2033.
Biomarkers Market : The global Biomarkers market size was estimated at USD 81.19 billion in 2023 and is projected to hit around USD 284.76 billion by 2033, growing at a CAGR of 13.37% during the forecast period from 2024 to 2033.
Clinical Trial Equipment & Ancillary Solutions Market : The global clinical trial equipment & ancillary solutions market size was exhibited at USD 2.99 billion in 2023 and is projected to hit around USD 7.01 billion by 2033, growing at a CAGR of 8.9% during the forecast period 2024 to 2033.
Clinical Trial Biorepository & Archiving Solutions Market : The global clinical trial biorepository & archiving solutions market size was exhibited at USD 4.70 billion in 2023 and is projected to hit around USD 10.27 billion by 2033, growing at a CAGR of 8.13% during the forecast period 2024 to 2033.
Clinical Trials Market : The global clinical trials market size was estimated at USD 81.90 billion in 2023 and is projected to hit around USD 153.59 billion by 2033, growing at a CAGR of 6.49% during the forecast period from 2024 to 2033.
Clinical Trial Supplies Market : The clinical trial supplies market size was exhibited at USD 3.55 billion in 2023 and is projected to hit around USD 8.49 billion by 2033, growing at a CAGR of 9.11% during the forecast period 2024 to 2033.
Clinical Trial Supply And Logistics Market : The global clinical trial supply and logistics market size was exhibited at USD 3.80 billion in 2023 and is projected to hit around USD 7.98 billion by 2033, growing at a CAGR of 7.7% during the forecast period of 2024 to 2033.
Clinical Trial Management Services Market : The global clinical trial management services market size was valued at USD 31.18 billion in 2023 and is anticipated to reach around USD 69.02 billion by 2033, growing at a CAGR of 8.27% from 2024 to 2033.
Clinical Trials Support Services Market : The global clinical trials support services market size was exhibited at USD 21.80 billion in 2023 and is projected to hit around USD 45.73 billion by 2033, growing at a CAGR of 7.69% during the forecast period 2024 to 2033.
Clinical Trial Imaging Market : The global clinical trial imaging market size was exhibited at USD 1.25 billion in 2023 and is projected to hit around USD 2.61 billion by 2033, growing at a CAGR of 7.65% during the forecast period 2024 to 2033.
Clinical Trial Kits Market: The global clinical trial kits market size was exhibited at USD 3.30 billion in 2023 and is projected to hit around USD 8.18 billion by 2033, growing at a CAGR of 9.5% during the forecast period 2024 to 2033.
Oncology Clinical Trials Market : The global oncology clinical trials market size reached USD 13.19 billion in 2023 and is projected to hit around USD 22.11 billion by 2033, expanding at a CAGR of 5.3% during the forecast period from 2024 to 2033.
Immuno-oncology Clinical Trials Market : The global immuno-oncology clinical trials market size was exhibited at USD 8.30 billion in 2023 and is projected to hit around USD 35.37 billion by 2033, growing at a CAGR of 15.6% during the forecast period 2024 to 2033.
Cell And Gene Therapy Clinical Trials Market : The global cell and gene therapy clinical trials market size reached USD 11.62 billion in 2023 and is projected to hit around USD 47.40 billion by 2033, expanding at a CAGR of 15.09% during the forecast period from 2024 to 2033.
eClinical Solutions Market : The global eclinical solutions market size was valued at USD 9.85 billion in 2023 and is projected to surpass around USD 37.16 billion by 2033, registering a CAGR of 14.2% over the forecast period of 2024 to 2033.
U.S. Clinical Trials Market : The U.S. clinical trials market size was valued at USD 25.81 billion in 2023 and is projected to surpass around USD 41.57 billion by 2033, registering a CAGR of 4.88% over the forecast period of 2024 to 2033.
U.S. Clinical Trial Imaging Market : The U.S. clinical trial imaging market size was valued at USD 409.50 million in 2023 and is projected to surpass around USD 875.93 million by 2033, registering a CAGR of 7.9% over the forecast period of 2024 to 2033.
U.S. Cell And Gene Therapy Clinical Trial Services Market : The U.S. cell and gene therapy clinical trial services market size was valued at USD 7.19 billion in 2023 and is projected to surpass around USD 56.53 billion by 2033, registering a CAGR of 22.9% over the forecast period of 2024 to 2033.
U.S. Pharmaceutical Market : The U.S. pharmaceutical market size was valued at USD 602.19 billion in 2023 and is projected to surpass around USD 1,093.79 billion by 2033, registering a CAGR of 6.15% over the forecast period of 2024 to 2033.
Call: USA: +1 650 460 3308 | IND: +91 87933 22019 |Europe: +44 2080772818
Web: https://www.novaoneadvisor.com/
You can place an order or ask any questions, please feel free to contact at sales@novaoneadvisor.com | +1 650 460 3308