Androgenetic Alopecia Market Outlook 2025-2035:
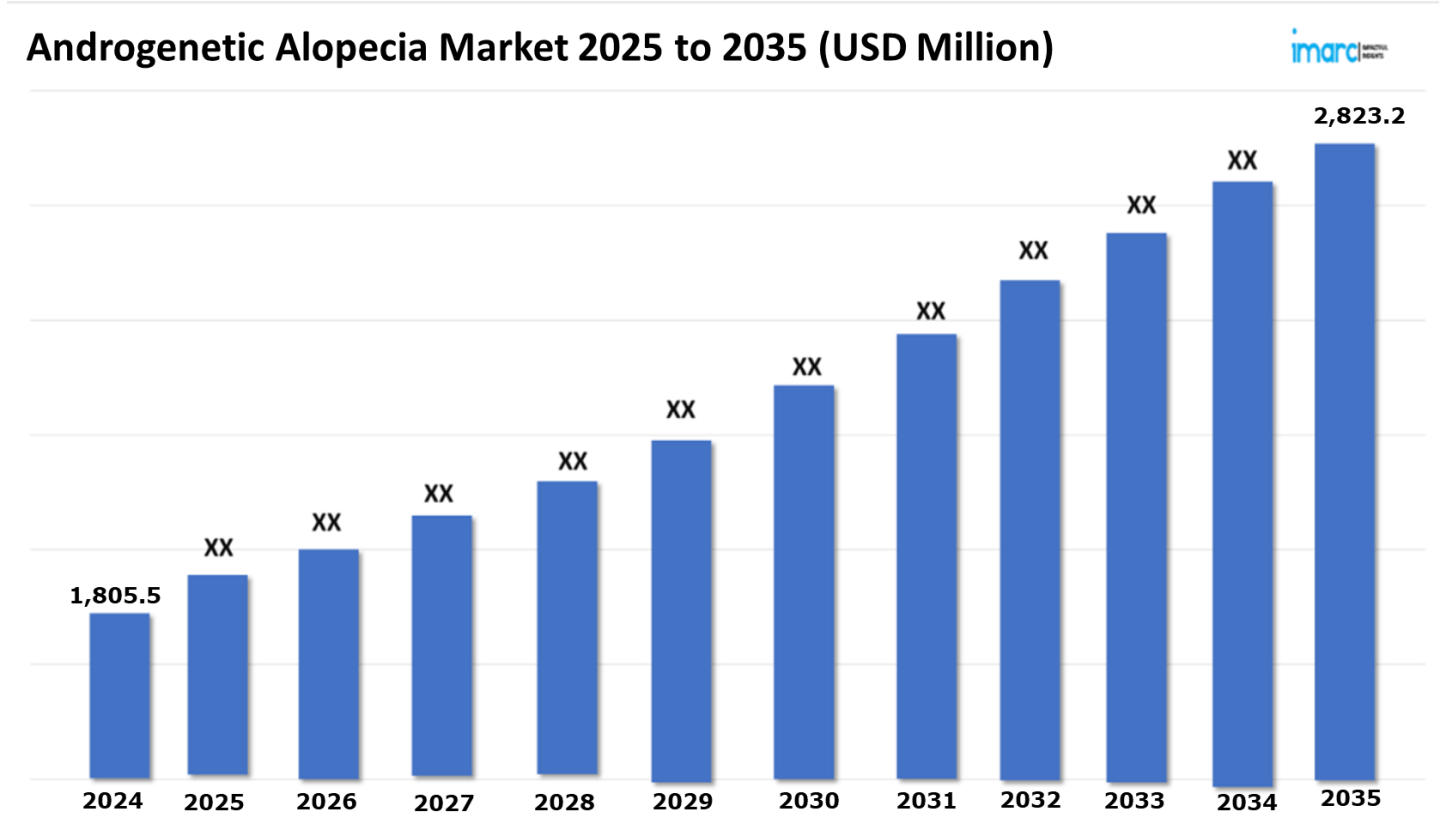
The 7 major androgenetic alopecia market reached a value of USD 1,805.5 Million in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 2,823.2 Million by 2035, exhibiting a growth rate (CAGR) of 4.2% during 2025-2035. The androgenetic alopecia market is driven by the rapidly increasing adoption of non-invasive and minimally invasive treatments, laser therapy, PRP therapy, and low-level laser therapy. These can help treat hair loss, reduce side effects, and minimize recovery periods. They are especially useful in stimulating hair growth, enhancing scalp health, and increasing the thickness and density of existing hair, thus resulting in better aesthetic outcomes and increased patient satisfaction. Such treatments reduce dependence on more invasive interventions such as hair transplants and long-term oral medications, thus making them an attractive alternative for those looking for efficient and convenient solutions to manage hair loss.
Advances in Early Detection and Diagnostic Technologies: Driving the Androgenetic Alopecia Market
Modern diagnostic and therapeutic technologies are significantly changing the androgenetic alopecia market, both in terms of management and patient outcomes. Advanced imaging modalities, such as scalp dermoscopy and high-resolution photography, enable precise visualization and monitoring of hair follicles, facilitating accurate assessments and personalized treatment strategies. These are accompanied by diagnostic tools such as blood tests for hormone levels and genetic profiling, which help identify the underlying causes of hair loss, such as hormonal imbalances or genetic factors.
Molecular diagnostics, including PCR and next-generation sequencing (NGS), are increasingly instrumental in detecting specific genetic markers, allowing for tailored treatment approaches. The integration of AI into diagnostic systems enables upgrading the precision of diagnosis by automating hair density analysis, assessment of the hair loss, and monitoring the course of treatment, which reduces reliance on subjective estimates. Non-invasive treatments such as low-level laser therapy, platelet-rich plasma therapy, and topical medications are popular mainly because these have shorter recovery times and lower side effects. Innovative wearable technologies, like smart devices monitoring the scalp health condition of the patient, provide real-time feedback on hair growth and treatment efficacy, enabling patients to manage their care at home. This is helpful for patients located in areas where it is difficult for them to access specialized dermatological care. Telemedicine platforms also play a progressively important role in expanding the availability of treatments for androgenetic alopecia, which can now include remote consultations, diagnoses, and treatment recommendations-all of which may be accomplished promptly and with as little inconvenience to the patient as possible from even the most inaccessible locations. New technologies are are making treatment more effective, accessible, and individualized.
Request a PDF Sample Report: https://www.imarcgroup.com/androgenetic-alopecia-market/requestsample
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The androgenetic alopecia market is being driven by new therapies that come with advanced pharmacological treatments. New topical and oral agents are being designed with specific genetic factors and hormonal imbalances as their focus, which puts them in the forefront for preventing and managing the condition. They are less prone to side effects, offer a targeted mechanism, and increase patient satisfaction and outcomes.
Biologic therapies research is gaining pace, especially in cases of moderate to severe androgenetic alopecia, commonly coupled with chronic hair thinning and loss. Some monoclonal antibodies focus on the action of pro-inflammatory cytokines like interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), acting directly on both hormonal and inflammatory hair loss aspects. These drugs can prevent inflammation-related mediators from harming the hair follicles, therefore encouraging new hair growth. Drug delivery technologies, including liposomal formulations, hydrogels, and nanotechnology-based carriers, are advancing to deliver treatment more targeted and localized. These systems concentrate therapeutic agents at the site of action, reducing systemic exposure and adverse effects. Adjunctive treatments under development to support scalp health and stimulate natural hair growth include probiotics, which restore the scalp microbiome, and immunomodulators that regulate the immune response. Combination therapies using topical or oral antimicrobials with anti-inflammatory agents or retinoids seem promising in managing the multifactorial etiopathogenesis of androgenetic alopecia. The popularity of non-invasive treatments, such as biofilm-disrupting agents and novel topical formulations, continues to grow for their patient-centric design, ease of use, and potential benefits on adherence. Such innovations transform the androgenetic alopecia market with improved accessible, better personal solutions, and efficiency to provide treatment options to patients dealing with hair loss.
Buy Full Report: https://www.imarcgroup.com/checkout?id=6640&method=809
Marketed Therapies in Androgenetic Alopecia Market
Minoxidil: Tritech Biopharmaceuticals
Minoxidil is a commonly used topical treatment for androgenetic alopecia that promotes hair growth and reduces hair loss by enhancing blood circulation to the hair follicles. It is typically regarded as a first-line treatment for both men and women, showing notable effectiveness in encouraging hair regrowth, especially in the crown region.
Finasteride: Merck & Co.
Finasteride is a commonly prescribed oral medication for treating androgenetic alopecia, especially in men. It functions by blocking the enzyme 5-alpha reductase, which decreases the conversion of testosterone to dihydrotestosterone (DHT), a hormone that contributes to the shrinking of hair follicles and hair loss. This action helps slow down hair loss and encourages hair regrowth in those affected.
Emerging Therapies in Androgenetic Alopecia Market
Dalosirvat: Biosplice Therapeutics
Dalosirvat is an investigational drug that targets the androgen receptor pathway, showing potential in treating Androgenetic Alopecia. By modulating androgenic activity, it aims to reduce hair loss and stimulate hair regrowth in individuals affected by this condition, offering a novel therapeutic approach.
Clascoterone: Cosmo Pharmaceuticals
Clascoterone is an innovative topical anti-androgenic treatment designed for androgenetic alopecia. It works by inhibiting the effects of androgens on hair follicles, helping to reduce hair loss and stimulate hair regrowth. Its localized action reduces the risk of systemic side effects, positioning it as a promising solution for androgen-related hair loss.
FOL 100: Follicle Pharma
FOL 100 is an experimental oral medication being developed to treat androgenetic alopecia. It targets key molecular pathways involved in hair loss, aiming to encourage hair regrowth by modulating androgen receptors and reducing the effect of dihydrotestosterone (DHT) on hair follicles. Initial studies have shown encouraging results in enhancing hair density and thickness.
Detailed list of emerging therapies in Androgenetic Alopecia is provided in the final report…
Leading Companies in the Androgenetic Alopecia Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global Androgenetic Alopecia market, several leading companies are at the forefront of developing integrated platforms to enhance the management of Androgenetic Alopecia. Some of the major players include Tritech Biopharmaceuticals, Merck & Co., Aclaris Therapeutics, and others. These companies are driving innovation in the Androgenetic Alopecia market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for Androgenetic Alopecia.
In February 2024, Technoderma Medicines announced data from a Phase IIa clinical trial of TDM-105795, its small-molecule drug candidate formulated as a topical solution for treating androgenetic alopecia. The randomized, double-blind study involved daily dosing over a four-month period and was designed to evaluate the solution's preliminary efficacy, pharmacokinetics, and safety. The trial enrolled 71 male participants at 13 clinical sites across the U.S., with subjects randomized in a 1:1:1 ratio.
Key Players in Androgenetic Alopecia Market:
The key players in the Androgenetic Alopecia market who are in different phases of developing different therapies are Tritech Biopharmaceuticals, Merck & Co., Aclaris Therapeutics, Biosplice Therapeutics, Dong-A ST, Follicle Pharma, and Others.
Regional Analysis:
The major markets for androgenetic alopecia include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for androgenetic alopecia while also representing the biggest market for its treatment. Recent advances in androgenetic alopecia treatment include the development of some innovative treatments. These advanced therapies include the advancement of small molecules, gene-modulating agents, anti-inflammatory compounds, and follicle-activating peptides, more precisely addressing androgen sensitivity, inflammation, and follicular miniaturization underlying mechanisms in patients with this kind of alopecia, bringing further improved outcomes without significantly increased adverse side effects.
Breakthroughs in molecular research have brought into the clinical scenario agents such as selective androgen receptor inhibitors (SARIs) and Wnt pathway activators, which are known to restore hair growth by reactivating the dormant follicles and promoting a healthier scalp environment. Bioactive compounds, such as microneedling with growth factors and platelet-rich plasma (PRP) enriched with stem cells, have further improved non-surgical treatment options for patients. Advances in diagnostic tools, such as AI-based imaging and genetic profiling, have facilitated earlier and more accurate identification of the stages of androgenetic alopecia and patient-specific treatment requirements. These developments have led to personalized, targeted interventions with minimal adverse events. Increased investment in research and development, along with increased collaboration among pharmaceutical companies, biotechnology firms, and research institutions, has further accelerated innovation in the androgenetic alopecia market. Telemedicine platforms and digital tools have made advanced treatments more accessible, bridging gaps for patients in remote and underserved areas, while the regions of North America and Europe continue to be leaders in innovation backed by the strength of their R&D pipelines, high adoption rates of new therapies, and a well-established healthcare infrastructure. With these innovations continuing to shape the market, the global androgenetic alopecia market is set for tremendous growth and promising developments in therapeutic and diagnostic domains.
Recent Developments in Androgenetic Alopecia Market:
· In August 2024, Pelage Pharmaceuticals, announced the initiation of its Phase 2a clinical trial with the first patients now dosed. The trial is focused on evaluating the safety and efficacy of PP405, an innovative topical small molecule designed to treat androgenetic alopecia (pattern baldness). The study plans to enroll 60 men and women and aims to investigate PP405’s potential to stimulate hair growth by reactivating dormant hair follicle stem cells.The study aims to enroll 60 men and women and is investigating PP405’s ability to reactivate dormant hair follicle stem cells, promoting hair growth.
· In January 2024, Sol-Gel Technologies, Ltd. announced that the Food and Drug Administration (FDA) has approved its first proprietary drug product, TWYNEO (tretinoin/benzoyl peroxide) cream, 0.1%/3%, indicated for the treatment of androgenetic alopecia in adults and pediatric patients nine years of age and older. TWYNEO utilizes Sol-Gel’s patented technology to encapsulate tretinoin, a retinoid, and benzoyl peroxide to stabilize tretinoin from degradation by benzoyl peroxide and to release each of the active drug ingredients slowly over time to provide a favorable safety profile and efficacy.
Key information covered in the report
Base Year: 2024
Historical Period: 2019-2024
Market Forecast: 2025-2035
Countries Covered
This report offers a comprehensive analysis of current Androgenetic Alopecia marketed drugs and late-stage pipeline drugs.
In-Market Drugs
IMARC Group Offer Other Reports:
Multiple Myeloma Market: The 7 major multiple myeloma markets reached a value of US$ 16.4 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 38.1 Billion by 2034, exhibiting a growth rate (CAGR) of 8% during 2024-2034.
Phenylketonuria Market: The 7 major phenylketonuria markets reached a value of US$ 956.5 Million in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 4,734.0 Million by 2034, exhibiting a growth rate (CAGR) of 15.65% during 2024-2034.
Polycythemia Vera Market: The 7 major polycythemia vera markets reached a value of US$ 17.1 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 68.5 Billion by 2034, exhibiting a growth rate (CAGR) of 13.44% during 2024-2034.
Spinal Muscular Atrophy Market: The 7 major spinal muscular atrophy markets reached a value of USD 3.4 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 14.3 Billion by 2035, exhibiting a growth rate (CAGR) of 13.81% during 2025-2035.
Substance Use Disorder Market: The 7 major substance use disorder markets reached a value of USD 5.3 Million in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 7.7 Million by 2035, exhibiting a growth rate (CAGR) of 3.51% during 2025-2035.
Venous Thromboembolism Market: The 7 major venous thromboembolism markets reached a value of US$ 3.8 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 7.0 Billion by 2034, exhibiting a growth rate (CAGR) of 5.71% during 2024-2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
The 7 major androgenetic alopecia market reached a value of USD 1,805.5 Million in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 2,823.2 Million by 2035, exhibiting a growth rate (CAGR) of 4.2% during 2025-2035. The androgenetic alopecia market is driven by the rapidly increasing adoption of non-invasive and minimally invasive treatments, laser therapy, PRP therapy, and low-level laser therapy. These can help treat hair loss, reduce side effects, and minimize recovery periods. They are especially useful in stimulating hair growth, enhancing scalp health, and increasing the thickness and density of existing hair, thus resulting in better aesthetic outcomes and increased patient satisfaction. Such treatments reduce dependence on more invasive interventions such as hair transplants and long-term oral medications, thus making them an attractive alternative for those looking for efficient and convenient solutions to manage hair loss.
Advances in Early Detection and Diagnostic Technologies: Driving the Androgenetic Alopecia Market
Modern diagnostic and therapeutic technologies are significantly changing the androgenetic alopecia market, both in terms of management and patient outcomes. Advanced imaging modalities, such as scalp dermoscopy and high-resolution photography, enable precise visualization and monitoring of hair follicles, facilitating accurate assessments and personalized treatment strategies. These are accompanied by diagnostic tools such as blood tests for hormone levels and genetic profiling, which help identify the underlying causes of hair loss, such as hormonal imbalances or genetic factors.
Molecular diagnostics, including PCR and next-generation sequencing (NGS), are increasingly instrumental in detecting specific genetic markers, allowing for tailored treatment approaches. The integration of AI into diagnostic systems enables upgrading the precision of diagnosis by automating hair density analysis, assessment of the hair loss, and monitoring the course of treatment, which reduces reliance on subjective estimates. Non-invasive treatments such as low-level laser therapy, platelet-rich plasma therapy, and topical medications are popular mainly because these have shorter recovery times and lower side effects. Innovative wearable technologies, like smart devices monitoring the scalp health condition of the patient, provide real-time feedback on hair growth and treatment efficacy, enabling patients to manage their care at home. This is helpful for patients located in areas where it is difficult for them to access specialized dermatological care. Telemedicine platforms also play a progressively important role in expanding the availability of treatments for androgenetic alopecia, which can now include remote consultations, diagnoses, and treatment recommendations-all of which may be accomplished promptly and with as little inconvenience to the patient as possible from even the most inaccessible locations. New technologies are are making treatment more effective, accessible, and individualized.
Request a PDF Sample Report: https://www.imarcgroup.com/androgenetic-alopecia-market/requestsample
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The androgenetic alopecia market is being driven by new therapies that come with advanced pharmacological treatments. New topical and oral agents are being designed with specific genetic factors and hormonal imbalances as their focus, which puts them in the forefront for preventing and managing the condition. They are less prone to side effects, offer a targeted mechanism, and increase patient satisfaction and outcomes.
Biologic therapies research is gaining pace, especially in cases of moderate to severe androgenetic alopecia, commonly coupled with chronic hair thinning and loss. Some monoclonal antibodies focus on the action of pro-inflammatory cytokines like interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), acting directly on both hormonal and inflammatory hair loss aspects. These drugs can prevent inflammation-related mediators from harming the hair follicles, therefore encouraging new hair growth. Drug delivery technologies, including liposomal formulations, hydrogels, and nanotechnology-based carriers, are advancing to deliver treatment more targeted and localized. These systems concentrate therapeutic agents at the site of action, reducing systemic exposure and adverse effects. Adjunctive treatments under development to support scalp health and stimulate natural hair growth include probiotics, which restore the scalp microbiome, and immunomodulators that regulate the immune response. Combination therapies using topical or oral antimicrobials with anti-inflammatory agents or retinoids seem promising in managing the multifactorial etiopathogenesis of androgenetic alopecia. The popularity of non-invasive treatments, such as biofilm-disrupting agents and novel topical formulations, continues to grow for their patient-centric design, ease of use, and potential benefits on adherence. Such innovations transform the androgenetic alopecia market with improved accessible, better personal solutions, and efficiency to provide treatment options to patients dealing with hair loss.
Buy Full Report: https://www.imarcgroup.com/checkout?id=6640&method=809
Marketed Therapies in Androgenetic Alopecia Market
Minoxidil: Tritech Biopharmaceuticals
Minoxidil is a commonly used topical treatment for androgenetic alopecia that promotes hair growth and reduces hair loss by enhancing blood circulation to the hair follicles. It is typically regarded as a first-line treatment for both men and women, showing notable effectiveness in encouraging hair regrowth, especially in the crown region.
Finasteride: Merck & Co.
Finasteride is a commonly prescribed oral medication for treating androgenetic alopecia, especially in men. It functions by blocking the enzyme 5-alpha reductase, which decreases the conversion of testosterone to dihydrotestosterone (DHT), a hormone that contributes to the shrinking of hair follicles and hair loss. This action helps slow down hair loss and encourages hair regrowth in those affected.
Emerging Therapies in Androgenetic Alopecia Market
Dalosirvat: Biosplice Therapeutics
Dalosirvat is an investigational drug that targets the androgen receptor pathway, showing potential in treating Androgenetic Alopecia. By modulating androgenic activity, it aims to reduce hair loss and stimulate hair regrowth in individuals affected by this condition, offering a novel therapeutic approach.
Clascoterone: Cosmo Pharmaceuticals
Clascoterone is an innovative topical anti-androgenic treatment designed for androgenetic alopecia. It works by inhibiting the effects of androgens on hair follicles, helping to reduce hair loss and stimulate hair regrowth. Its localized action reduces the risk of systemic side effects, positioning it as a promising solution for androgen-related hair loss.
FOL 100: Follicle Pharma
FOL 100 is an experimental oral medication being developed to treat androgenetic alopecia. It targets key molecular pathways involved in hair loss, aiming to encourage hair regrowth by modulating androgen receptors and reducing the effect of dihydrotestosterone (DHT) on hair follicles. Initial studies have shown encouraging results in enhancing hair density and thickness.
| Drug Name | Company Name | MOA | ROA |
| Dalosirvat | Biosplice Therapeutics | Wnt signalling pathway stimulants | Topical |
| Clascoterone | Cosmo Pharmaceuticals | Androgen receptor antagonists | Topical |
| FOL 100 | Follicle Pharma | Dihydrotestosterone (DHT) blockers | Topical |
Leading Companies in the Androgenetic Alopecia Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global Androgenetic Alopecia market, several leading companies are at the forefront of developing integrated platforms to enhance the management of Androgenetic Alopecia. Some of the major players include Tritech Biopharmaceuticals, Merck & Co., Aclaris Therapeutics, and others. These companies are driving innovation in the Androgenetic Alopecia market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for Androgenetic Alopecia.
In February 2024, Technoderma Medicines announced data from a Phase IIa clinical trial of TDM-105795, its small-molecule drug candidate formulated as a topical solution for treating androgenetic alopecia. The randomized, double-blind study involved daily dosing over a four-month period and was designed to evaluate the solution's preliminary efficacy, pharmacokinetics, and safety. The trial enrolled 71 male participants at 13 clinical sites across the U.S., with subjects randomized in a 1:1:1 ratio.
Key Players in Androgenetic Alopecia Market:
The key players in the Androgenetic Alopecia market who are in different phases of developing different therapies are Tritech Biopharmaceuticals, Merck & Co., Aclaris Therapeutics, Biosplice Therapeutics, Dong-A ST, Follicle Pharma, and Others.
Regional Analysis:
The major markets for androgenetic alopecia include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for androgenetic alopecia while also representing the biggest market for its treatment. Recent advances in androgenetic alopecia treatment include the development of some innovative treatments. These advanced therapies include the advancement of small molecules, gene-modulating agents, anti-inflammatory compounds, and follicle-activating peptides, more precisely addressing androgen sensitivity, inflammation, and follicular miniaturization underlying mechanisms in patients with this kind of alopecia, bringing further improved outcomes without significantly increased adverse side effects.
Breakthroughs in molecular research have brought into the clinical scenario agents such as selective androgen receptor inhibitors (SARIs) and Wnt pathway activators, which are known to restore hair growth by reactivating the dormant follicles and promoting a healthier scalp environment. Bioactive compounds, such as microneedling with growth factors and platelet-rich plasma (PRP) enriched with stem cells, have further improved non-surgical treatment options for patients. Advances in diagnostic tools, such as AI-based imaging and genetic profiling, have facilitated earlier and more accurate identification of the stages of androgenetic alopecia and patient-specific treatment requirements. These developments have led to personalized, targeted interventions with minimal adverse events. Increased investment in research and development, along with increased collaboration among pharmaceutical companies, biotechnology firms, and research institutions, has further accelerated innovation in the androgenetic alopecia market. Telemedicine platforms and digital tools have made advanced treatments more accessible, bridging gaps for patients in remote and underserved areas, while the regions of North America and Europe continue to be leaders in innovation backed by the strength of their R&D pipelines, high adoption rates of new therapies, and a well-established healthcare infrastructure. With these innovations continuing to shape the market, the global androgenetic alopecia market is set for tremendous growth and promising developments in therapeutic and diagnostic domains.
Recent Developments in Androgenetic Alopecia Market:
· In August 2024, Pelage Pharmaceuticals, announced the initiation of its Phase 2a clinical trial with the first patients now dosed. The trial is focused on evaluating the safety and efficacy of PP405, an innovative topical small molecule designed to treat androgenetic alopecia (pattern baldness). The study plans to enroll 60 men and women and aims to investigate PP405’s potential to stimulate hair growth by reactivating dormant hair follicle stem cells.The study aims to enroll 60 men and women and is investigating PP405’s ability to reactivate dormant hair follicle stem cells, promoting hair growth.
· In January 2024, Sol-Gel Technologies, Ltd. announced that the Food and Drug Administration (FDA) has approved its first proprietary drug product, TWYNEO (tretinoin/benzoyl peroxide) cream, 0.1%/3%, indicated for the treatment of androgenetic alopecia in adults and pediatric patients nine years of age and older. TWYNEO utilizes Sol-Gel’s patented technology to encapsulate tretinoin, a retinoid, and benzoyl peroxide to stabilize tretinoin from degradation by benzoyl peroxide and to release each of the active drug ingredients slowly over time to provide a favorable safety profile and efficacy.
Key information covered in the report
Base Year: 2024
Historical Period: 2019-2024
Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the Androgenetic Alopecia market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the Androgenetic Alopecia market
- Reimbursement scenario in the market
- In-market and pipeline drugs
This report offers a comprehensive analysis of current Androgenetic Alopecia marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
IMARC Group Offer Other Reports:
Multiple Myeloma Market: The 7 major multiple myeloma markets reached a value of US$ 16.4 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 38.1 Billion by 2034, exhibiting a growth rate (CAGR) of 8% during 2024-2034.
Phenylketonuria Market: The 7 major phenylketonuria markets reached a value of US$ 956.5 Million in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 4,734.0 Million by 2034, exhibiting a growth rate (CAGR) of 15.65% during 2024-2034.
Polycythemia Vera Market: The 7 major polycythemia vera markets reached a value of US$ 17.1 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 68.5 Billion by 2034, exhibiting a growth rate (CAGR) of 13.44% during 2024-2034.
Spinal Muscular Atrophy Market: The 7 major spinal muscular atrophy markets reached a value of USD 3.4 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 14.3 Billion by 2035, exhibiting a growth rate (CAGR) of 13.81% during 2025-2035.
Substance Use Disorder Market: The 7 major substance use disorder markets reached a value of USD 5.3 Million in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 7.7 Million by 2035, exhibiting a growth rate (CAGR) of 3.51% during 2025-2035.
Venous Thromboembolism Market: The 7 major venous thromboembolism markets reached a value of US$ 3.8 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 7.0 Billion by 2034, exhibiting a growth rate (CAGR) of 5.71% during 2024-2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800