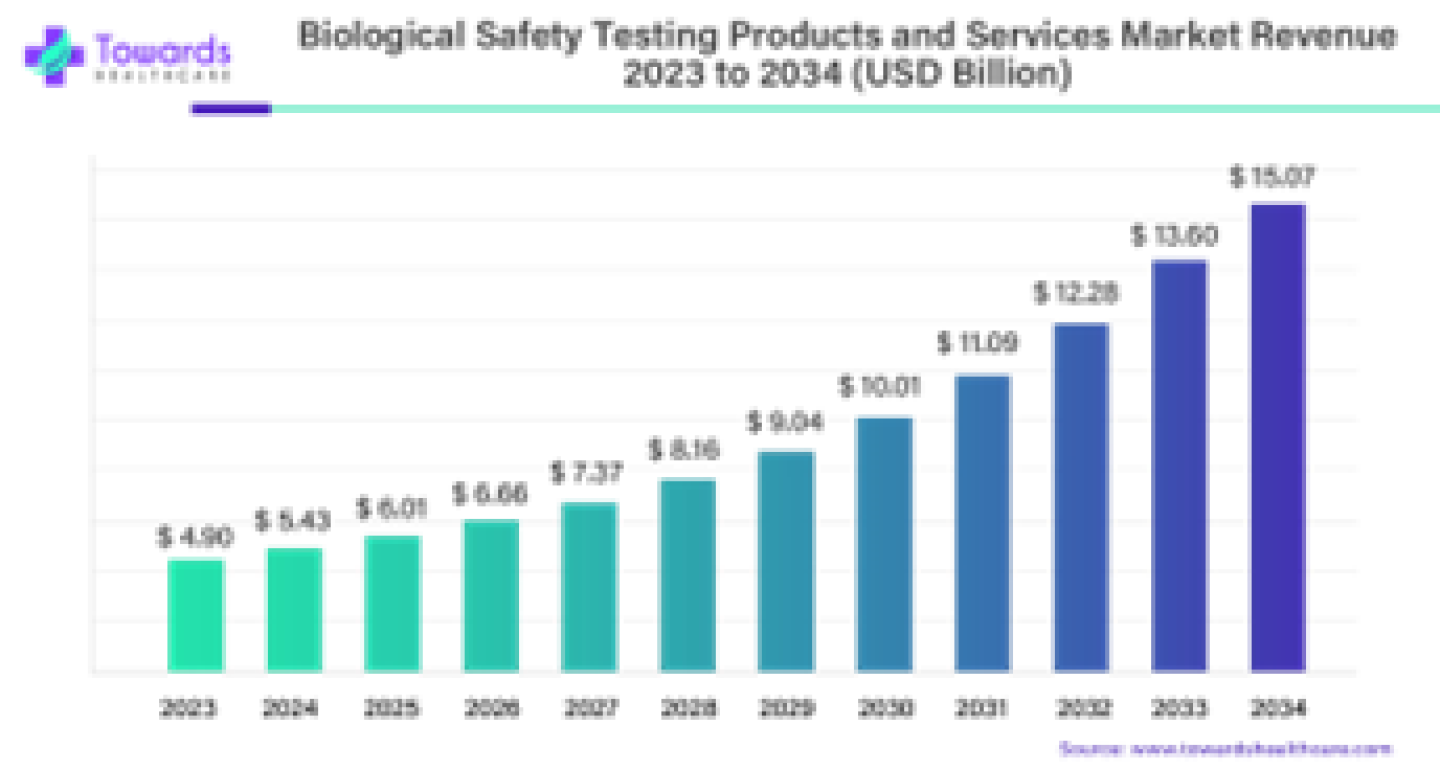
The global biological safety testing products and services market size surpassed USD 4.90 billion in 2023 and is projected to reach around USD 15.07 billion by 2034, expanding at a CAGR of 10.75% from 2024 to 2034.
Download a sample version of this report @ https://www.towardshealthcare.com/personalized-scope/5209
In 2024, market is to achieve USD 5.43 billion with rising prevalence of infectious diseases. The need for effective safety measures are driving the market growth. The increasing focus on public health and the development of new biological products are contributing to the expansion of the market.
Major Key Insights of the Biological Safety Testing Products and Services Market
Biological Safety Testing Products and Services Market: A Crucial Safeguard
The biological safety testing products and services market involves the companies and labs that provide these testing products and services. In recent years there is increasing demand for biologics like vaccines, gene therapies and other advanced treatments which is significantly driving the biological safety testing products and services market. With strict regulations from the government health agency, manufacturers are required to perform thorough safety checks which boosts the need for reliable biological safety testing services.
Due to factors like the rise of new diseases, increased production of biologic drugs and a stronger focus on quality and safety the market has seen increased growth. As biotechnology continues to expand in coming years, the demand for biological safety testing products and services will keep rising ensuring that new treatments are safe and effective for patients all over the globe.
Recent Developments in Biological Safety Testing Products and Services:
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Top Companies in the Biological Safety Testing Products and Services Market
· Charles River Laboratories
· Lonza
· BIOMÉRIEUX
· SGS Société Générale de Surveillance SA
· Eurofins Scientific
· Samsung Biologics
· FUJIFILM Wako Pure Chemical Corporation
· Thermo Fisher Scientific Inc.
· Sartorius AG
· BSL Bioservice
· Merck KGaA
Driver
Growing Emphasis on R&D Fuels Market Expansion
There has been a significant rise in investments directed towards research and development in recent years, within the pharmaceutical and biotechnology industries. These investments were specially made in the development of biologics and biosimilars. This surge in financial backing is acting as a key driver in the expansion of the biological safety testing products and services market, as companies are increasingly focusing on creating innovative biological products. These advancements in biologics require comprehensive safety testing to comply with stringent regulatory guidelines set by health authorities like FDA, EMA and others.
Recent R&D Investments by Key Biologics Companies 2022-2023:
Customize this study as per your requirement @ https://www.towardshealthcare.com/customization/5209
Restraint
High Cost of Biological Safety Testing Restraining the Market
The high cost associated with conducting comprehensive safety evaluations is one of the major hurdles in the biological safety testing products and services market. These procedures are not only essential but also require advanced and specialized equipment such as state-of-the-art analytical instruments, Containment facilities and sterile environments. Maintaining these equipment’s comes with the hefty price and significant expenses, which makes the process costly for many organizations particularly smaller biotechnology firms and startups. This cost factor often becomes a barrier which limits innovation in the sector.
Customization and Outsourcing, An Emerging Opportunity
In recent years customization has emerged as a significant opportunity for the growth post up biological safety testing products and services market. With time more businesses are seeking tailored solutions to meet their specific needs, whether it's the type of samples being tested or unique regulatory considerations. Customization involves adjusting testing services to suit individual client demand, ranging from assay development to modifying testing methodologies to handle complex or atypical sample types. This approach allows companies to ensure their products meet the exact safety standards which are required for innovative therapies like cell and gene treatments.
By product of biological safety testing products and services market, the reagent and kits segment held the largest share of the market in 2023. These products are essential for conducting various safety tests like endotoxin, bioburden, and sterility test. Reagents and kits are widely used because they provide consistency, convenience skin testing and accuracy.
By the application of biological safety testing products and services market the vaccine and therapeutic segment dominated the market in 2023 while the gene therapy segment is expected to grow at the fastest rate during the forecast time. The ongoing development of new vaccines such as mRNA vaccine for COVID-19 and other infectious diseases has significantly boosted vaccines and therapeutic segment. Companies are required to perform rigorous biological safety tests to meet regulatory standards.
For instance, in June of 2023, Moderna, expanded its vaccine development pipeline, requiring extensive biological safety testing to ensure the safety of its new products. This constant development of vaccines ensures the dominance of this segment.
Endotoxin Tests Segment Held the Dominant Share of the Market
Endotoxin tests, dominated by type segment of biological safety testing products and services market in 2023. These tests are crucial for ensuring that products like therapeutic drugs, vaccines and other biologics are free from harmful bacterial toxins. The importance of endotoxin testing was emphasized when in February 2023, Lonza, which is a leading pharmaceutical company introduced new endotoxin testing methods to speed up safety evaluations in their production line. The need for consistent and reliable testing methods across the pharmaceutical industry is driving this dominance.
Drug Manufacturing Sites, 2022:
Pharma Giants Lead North American Market in 2023
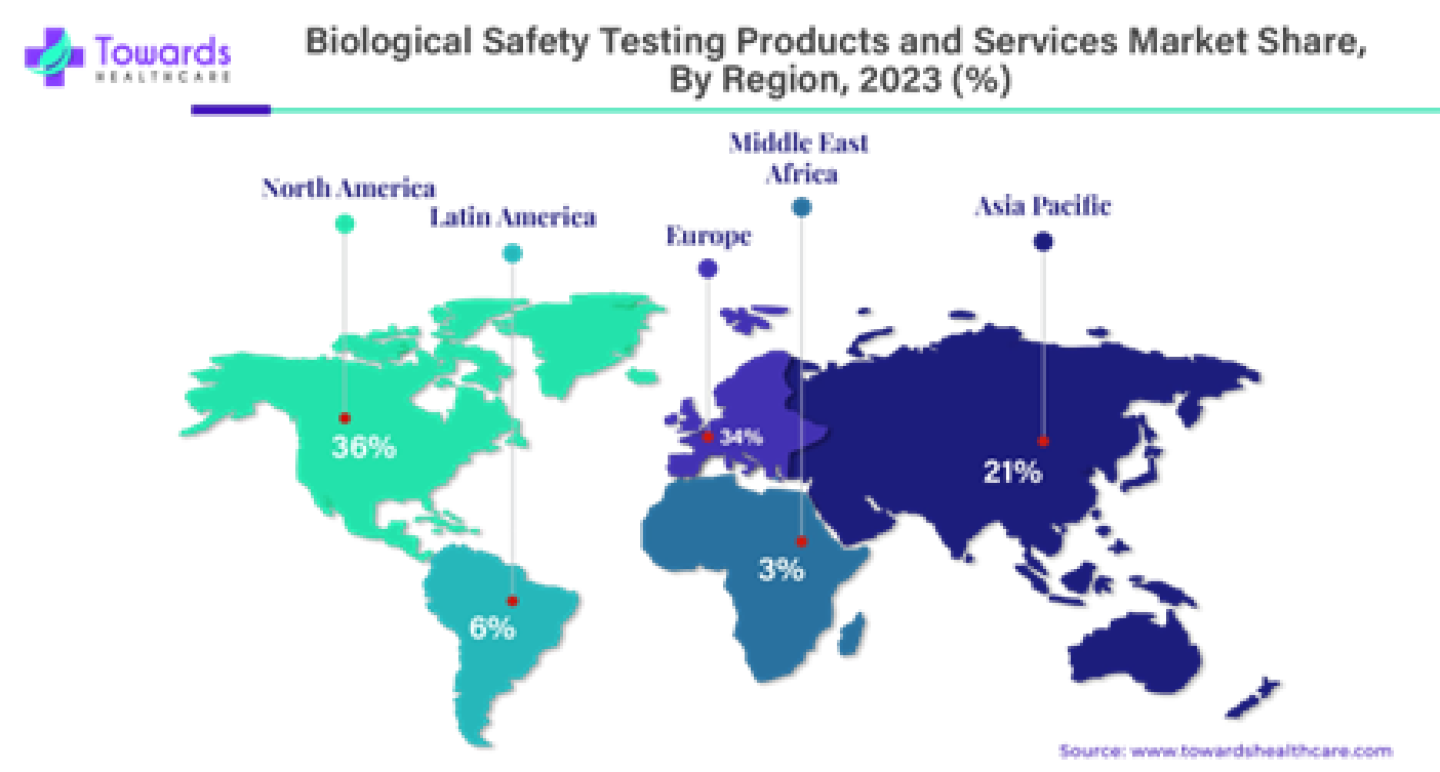
The region North America dominated the biological safety testing products and services market in 2023 accounting for approximately 36% of the global market share. The country United States plays a crucial role in this dominance. The United States has robust pharmaceutical industry and cutting-edge healthcare infrastructure which is driving this dominance. The US has witnessed significant advancements in biotechnology, including the introduction of automated biological safety testing methods which improve testing accuracy while minimizing human intervention. This has streamlined processes for pharmaceutical companies and research institutions making the US a leader in the biological safety testing products and services market.
Government Support Boosts Asis Pacific Pharma Industry
The region Asia Pacific is projected to grow at the fastest rate in the biologic safety testing products and services market during forecast time. Rising population and increasing demand for safe and high-quality therapeutics across the region are some factors contributing to this rapid growth. Countries like China, Japan and India are at the forefront of this expansion.
The Indian government has launched several initiatives to support the pharmaceutical industry and improve public health, that's why India is also a significant player in the region and is experiencing a surge in the production of therapeutic drugs and vaccines.
Browse More Insights of Towards Healthcare:
Spine Biologics Market: https://www.towardshealthcare.com/insights/spine-biologics-market-sizing
Cancer Biologics Market: https://www.towardshealthcare.com/insights/cancer-biologics-market-sizing
Retinal Biologics Market: https://www.towardshealthcare.com/insights/retinal-biologics-market-sizing
Biobanking Market: https://www.towardshealthcare.com/insights/biobanking-market-sizing
Computational Biology Market: https://www.towardshealthcare.com/insights/computational-biology-market-sizing
Biomaterials Market: https://www.towardshealthcare.com/insights/biomaterials-market-sizing
Biotechnology Instruments Market: https://www.towardshealthcare.com/insights/biotechnology-instruments-market-sizing
Clinical Microbiology Market: https://www.towardshealthcare.com/insights/clinical-microbiology-market-sizing
Biosimilars Market: https://www.towardshealthcare.com/insights/biosimilars-market
Liquid Biopsy Market: https://www.towardshealthcare.com/insights/liquid-biopsy-an-emerging-cancer-diagnostic
Segments Covered in the Report
By Product
· Executive Summary
· Introduction
· Market Dynamics
· Regulatory Landscape
· Biological Safety Testing Market Overview
· Top Companies in the Biological Safety Testing Products and Services Market
· Segmentation Analysis
· Cross Segment Analysis
· Go-to-Market Strategies
· Integration of AI in the Biological Safety Testing Products and Services Market
· Production and Consumption in the Market
· Strategic Components
· Competitive Landscape
· Emerging Trends in Biological Safety Testing
· Market Opportunities and Future Outlook
· Appendix
View full TOC @ https://www.towardshealthcare.com/table-of-content/biological-safety-testing-products-and-services-market-sizing
Acquire our comprehensive analysis today @ https://www.towardshealthcare.com/price/5209
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Gain access to the latest insights and statistics in the healthcare industry by subscribing to our Annual Membership. Stay updated on healthcare industry segmentation with detailed reports, market trends, and expert analysis tailored to your needs. Stay ahead of the curve with valuable resources and strategic recommendations. Join today to unlock a wealth of knowledge and opportunities in the dynamic world of healthcare: Get a Subscription
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations. We are a global strategy consulting firm that assists business leaders in gaining a competitive edge and accelerating growth. We are a provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations.
Browse our Brand-New Journals:
https://www.towardspackaging.com
https://www.towardsautomotive.com
https://www.precedenceresearch.com
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare
Get Our Freshly Printed Chronicle: https://www.healthcarewebwire.com
Download a sample version of this report @ https://www.towardshealthcare.com/personalized-scope/5209
In 2024, market is to achieve USD 5.43 billion with rising prevalence of infectious diseases. The need for effective safety measures are driving the market growth. The increasing focus on public health and the development of new biological products are contributing to the expansion of the market.
Major Key Insights of the Biological Safety Testing Products and Services Market
- North America dominated the market share by 36% in 2023.
- Asia Pacific is expected to grow at the fastest rate during the forecast period.
- By product, the reagent & kits segment held the largest share of the market in 2023.
- By application, the vaccines and therapeutics segment dominated the biological safety testing products and services market in 2023.
- By application, the gene therapy segment is expected to grow at the fastest rate during the forecast period.
- By test type, the endotoxin tests segment held the dominant share of the market in 2023.
Biological Safety Testing Products and Services Market: A Crucial Safeguard
The biological safety testing products and services market involves the companies and labs that provide these testing products and services. In recent years there is increasing demand for biologics like vaccines, gene therapies and other advanced treatments which is significantly driving the biological safety testing products and services market. With strict regulations from the government health agency, manufacturers are required to perform thorough safety checks which boosts the need for reliable biological safety testing services.
Due to factors like the rise of new diseases, increased production of biologic drugs and a stronger focus on quality and safety the market has seen increased growth. As biotechnology continues to expand in coming years, the demand for biological safety testing products and services will keep rising ensuring that new treatments are safe and effective for patients all over the globe.
Recent Developments in Biological Safety Testing Products and Services:
| Company Name | Sartorius |
| Headquarters | Gottingen, Germany, Europe |
| Development | In May 2024, a major advancement in the field of sterility testing was recently revealed by Sartorius, a life science organization, with the introduction of the fourth generation Sterisart® Universal pump. This innovative pump satisfies the exact quality and safety requirements required by the pharmaceutical and biotechnology industries of today. It is designed to transfer samples and media into Sterisart® canisters in a dependable manner. |
| Company Name | Charles River Laboratories International |
| Headquarters | Massachusetts, United States, North America |
| Development | In January 2024, Charles River Laboratories International, Inc. developed the Endosafe® TrilliumTM rCR cartridge offering by combining their recombinant cascade reagent (rCR) with their flagship Endosafe® cartridge technology. Adding a new testing method that doesn't use animals to its extensive line of bacterial endotoxin testing (BET) products. |
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Top Companies in the Biological Safety Testing Products and Services Market
· Charles River Laboratories
· Lonza
· BIOMÉRIEUX
· SGS Société Générale de Surveillance SA
· Eurofins Scientific
· Samsung Biologics
· FUJIFILM Wako Pure Chemical Corporation
· Thermo Fisher Scientific Inc.
· Sartorius AG
· BSL Bioservice
· Merck KGaA
Driver
Growing Emphasis on R&D Fuels Market Expansion
There has been a significant rise in investments directed towards research and development in recent years, within the pharmaceutical and biotechnology industries. These investments were specially made in the development of biologics and biosimilars. This surge in financial backing is acting as a key driver in the expansion of the biological safety testing products and services market, as companies are increasingly focusing on creating innovative biological products. These advancements in biologics require comprehensive safety testing to comply with stringent regulatory guidelines set by health authorities like FDA, EMA and others.
Recent R&D Investments by Key Biologics Companies 2022-2023:
| Company | R&D Investment (2022) | R&D Investment (2023) | Focus Area | Impact on Safety Testing |
| Amgen | USD 1.3 Billion | USD 1.5 Billion | biologics and biosimilars | increase demand for biosafety testing services |
| Pfizer | USD 10.5 Billion | USD 11.4 Billion | vaccine development and biologics | extensive testing for vaccine safety and efficacy |
| Roche | USD 14.6 Billion | USD 15 Billion | cancer and biologics therapies | higher safety testing for novel cancer therapies |
| Moderna | USD 2.1 Billion | USD 2.7 Billion | mRNA vaccines and therapies | significant increase in mRNA safety testing needs |
| Novartis | USD 9.4 Billion | USD 9.6 Billion | gene therapies and biologics | enhanced testing for gene-editing technologies |
Customize this study as per your requirement @ https://www.towardshealthcare.com/customization/5209
Restraint
High Cost of Biological Safety Testing Restraining the Market
The high cost associated with conducting comprehensive safety evaluations is one of the major hurdles in the biological safety testing products and services market. These procedures are not only essential but also require advanced and specialized equipment such as state-of-the-art analytical instruments, Containment facilities and sterile environments. Maintaining these equipment’s comes with the hefty price and significant expenses, which makes the process costly for many organizations particularly smaller biotechnology firms and startups. This cost factor often becomes a barrier which limits innovation in the sector.
- For instance, in May of 2023, a report form Bio Reliance (Merck Group) Noted that the average cost 4 routine biological safety tests have surged by nearly 20% in the last five years, which is mainly due to the rising complexity and by pharmaceutical products and the stringent regulatory requirements.
Customization and Outsourcing, An Emerging Opportunity
In recent years customization has emerged as a significant opportunity for the growth post up biological safety testing products and services market. With time more businesses are seeking tailored solutions to meet their specific needs, whether it's the type of samples being tested or unique regulatory considerations. Customization involves adjusting testing services to suit individual client demand, ranging from assay development to modifying testing methodologies to handle complex or atypical sample types. This approach allows companies to ensure their products meet the exact safety standards which are required for innovative therapies like cell and gene treatments.
- A leading biotechnology company called Bluebird Bio, in 2022, developed a customized viral vector safety testing protocol in partnership with contract research organization (CRO). This collaboration allowed them to meet stringent FDA guidelines for gene therapy products.
By product of biological safety testing products and services market, the reagent and kits segment held the largest share of the market in 2023. These products are essential for conducting various safety tests like endotoxin, bioburden, and sterility test. Reagents and kits are widely used because they provide consistency, convenience skin testing and accuracy.
- In April 2023, Bioburden and Sterility Testing services for medical devices are now offered by STEMart, a U.S.-based company that offers complete services for all phases of medical device development, following the guidelines of ISO 11731.
By the application of biological safety testing products and services market the vaccine and therapeutic segment dominated the market in 2023 while the gene therapy segment is expected to grow at the fastest rate during the forecast time. The ongoing development of new vaccines such as mRNA vaccine for COVID-19 and other infectious diseases has significantly boosted vaccines and therapeutic segment. Companies are required to perform rigorous biological safety tests to meet regulatory standards.
For instance, in June of 2023, Moderna, expanded its vaccine development pipeline, requiring extensive biological safety testing to ensure the safety of its new products. This constant development of vaccines ensures the dominance of this segment.
Endotoxin Tests Segment Held the Dominant Share of the Market
Endotoxin tests, dominated by type segment of biological safety testing products and services market in 2023. These tests are crucial for ensuring that products like therapeutic drugs, vaccines and other biologics are free from harmful bacterial toxins. The importance of endotoxin testing was emphasized when in February 2023, Lonza, which is a leading pharmaceutical company introduced new endotoxin testing methods to speed up safety evaluations in their production line. The need for consistent and reliable testing methods across the pharmaceutical industry is driving this dominance.
Drug Manufacturing Sites, 2022:
Pharma Giants Lead North American Market in 2023
The region North America dominated the biological safety testing products and services market in 2023 accounting for approximately 36% of the global market share. The country United States plays a crucial role in this dominance. The United States has robust pharmaceutical industry and cutting-edge healthcare infrastructure which is driving this dominance. The US has witnessed significant advancements in biotechnology, including the introduction of automated biological safety testing methods which improve testing accuracy while minimizing human intervention. This has streamlined processes for pharmaceutical companies and research institutions making the US a leader in the biological safety testing products and services market.
- In 2022, The United States healthcare system received a boost with increased research and development spending which enhanced the development and safety testing of new drugs and therapies.
Government Support Boosts Asis Pacific Pharma Industry
The region Asia Pacific is projected to grow at the fastest rate in the biologic safety testing products and services market during forecast time. Rising population and increasing demand for safe and high-quality therapeutics across the region are some factors contributing to this rapid growth. Countries like China, Japan and India are at the forefront of this expansion.
The Indian government has launched several initiatives to support the pharmaceutical industry and improve public health, that's why India is also a significant player in the region and is experiencing a surge in the production of therapeutic drugs and vaccines.
- For instance, the first mobile biosafety Level 3 laboratory was launched in Nasik city of Maharashtra region in India, in February of 2022. This mobile lab allows researchers to study highly contagious and deadly viral diseases which is enhancing the country's capacity for biological safety testing.
Browse More Insights of Towards Healthcare:
Spine Biologics Market: https://www.towardshealthcare.com/insights/spine-biologics-market-sizing
Cancer Biologics Market: https://www.towardshealthcare.com/insights/cancer-biologics-market-sizing
Retinal Biologics Market: https://www.towardshealthcare.com/insights/retinal-biologics-market-sizing
Biobanking Market: https://www.towardshealthcare.com/insights/biobanking-market-sizing
Computational Biology Market: https://www.towardshealthcare.com/insights/computational-biology-market-sizing
Biomaterials Market: https://www.towardshealthcare.com/insights/biomaterials-market-sizing
Biotechnology Instruments Market: https://www.towardshealthcare.com/insights/biotechnology-instruments-market-sizing
Clinical Microbiology Market: https://www.towardshealthcare.com/insights/clinical-microbiology-market-sizing
Biosimilars Market: https://www.towardshealthcare.com/insights/biosimilars-market
Liquid Biopsy Market: https://www.towardshealthcare.com/insights/liquid-biopsy-an-emerging-cancer-diagnostic
Segments Covered in the Report
By Product
- Reagents & Kits
- Instruments
- Services
- Vaccines & Therapeutics
- Vaccines
- Monoclonal Antibodies
- Recombinant Protein
- Gene Therapy
- Blood & Blood-based Products
- Tissue & Tissue-based Products
- Stem Cell
- Endotoxin Tests
- Bioburden Tests
- Sterility Tests
- Cell Line Authentication & Characterization Tests
- Adventitious Agent Detection Tests
- Residual Host Contamination Detection Tests
- Others
- North America
- U.S.
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Thailands
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Sweden
- Denmark
- Norway
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa (MEA)
- South Africa
- UAE
- Saudi Arabia
- Kuwait
· Executive Summary
· Introduction
· Market Dynamics
· Regulatory Landscape
· Biological Safety Testing Market Overview
· Top Companies in the Biological Safety Testing Products and Services Market
· Segmentation Analysis
· Cross Segment Analysis
· Go-to-Market Strategies
· Integration of AI in the Biological Safety Testing Products and Services Market
· Production and Consumption in the Market
· Strategic Components
· Competitive Landscape
· Emerging Trends in Biological Safety Testing
· Market Opportunities and Future Outlook
· Appendix
View full TOC @ https://www.towardshealthcare.com/table-of-content/biological-safety-testing-products-and-services-market-sizing
Acquire our comprehensive analysis today @ https://www.towardshealthcare.com/price/5209
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Gain access to the latest insights and statistics in the healthcare industry by subscribing to our Annual Membership. Stay updated on healthcare industry segmentation with detailed reports, market trends, and expert analysis tailored to your needs. Stay ahead of the curve with valuable resources and strategic recommendations. Join today to unlock a wealth of knowledge and opportunities in the dynamic world of healthcare: Get a Subscription
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations. We are a global strategy consulting firm that assists business leaders in gaining a competitive edge and accelerating growth. We are a provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations.
Browse our Brand-New Journals:
https://www.towardspackaging.com
https://www.towardsautomotive.com
https://www.precedenceresearch.com
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare
Get Our Freshly Printed Chronicle: https://www.healthcarewebwire.com