Cardiomyopathy Market Outlook 2025-2035:
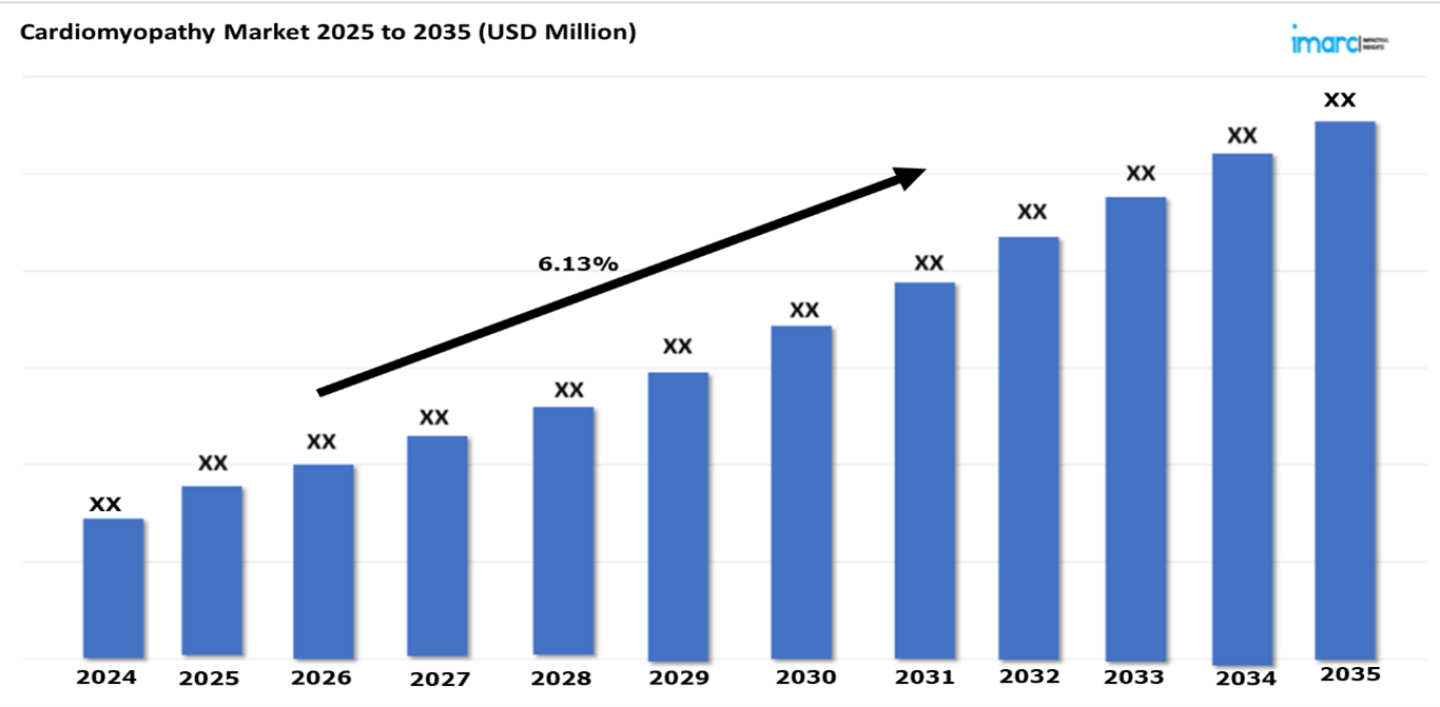
The 7 majors cardiomyopathy market are expected to exhibit a CAGR of 6.13% during 2025-2035. The cardiomyopathy market is seeing rapid growth because of awareness related to cardiovascular diseases and rising lifestyles of disease risks such as obesity, diabetes, and hypertension. Diagnostic technology improvement, novel therapeutic drug discoveries, and the use of genetic tests are fueling expansion in this market. A rising level of healthcare spending, aged populations, and initiatives from government organizations towards enhanced cardiovascular health are raising demand. New treatments like gene and cell therapies open significant opportunities for market players. Innovation is further accelerated by partnerships between pharmaceutical companies and research institutions, enhancing the growth trajectory of the market.
Rising Prevalence of Cardiovascular Diseases: Driving the Cardiomyopathy Market
The major driver of the growth of the cardiomyopathy market is the increasing rates of cardiovascular diseases. Cardiomyopathy is a disorder affecting the muscular structure of the heart. It is usually linked with certain cardiovascular conditions or diseases, such as coronary artery disease or heart failure. Many factors that contribute to these diseases include rapid urbanization, physical inactivity, and unhealthy diets that prompt obesity, diabetes, and hypercholesterolemia. Echocardiography and cardiac MRI are employed more frequently as they enable improvement and enhanced detection at early stages. There are also cutting-edge treatment options, gene therapy, targeted medicines, implantable devices- pacemakers and defibrillators in support of treatment, thereby creating an impetus for the expansion of the market. Wide-scale awareness programs on cardiomyopathy and funding made by both governments and private persons for its study also promote this type of growth in the market. The United States, Europe, and parts of Asia are the countries with high rates of cardiovascular diseases; they are experiencing an increase in demand for cardiomyopathy-related products and services. The growing prevalence of cardiovascular diseases drives the market for cardiomyopathy.
Request a PDF Sample Report: https://www.imarcgroup.com/cardiomyopathy-market/requestsample
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
One of the major positive drivers for the market has been the development of new therapies and pharmacological interventions for cardiomyopathy. Traditionally, drugs like beta-blockers, ACE inhibitors, and diuretics were used to treat these ailments on account of the weakening and dilatation of the heart muscle. Gene therapies, cell-based treatments, and advanced pharmacological interventions represent some of the new and innovative treatment options. Apart from gene therapies and immunotherapies, scientists also consider the repair of DNA mutation with CRISPR and other genetic engineering tools. Simultaneously, cell therapies from stem cells, along with regenerative medicines, may provide tissue regeneration in cases of damaged heart tissue. Stem cell therapy aims to reduce the course of the disease, further raising the patients’ survival ratio. Also, the treatment of molecular targets or pathways, like cardiac myosin inhibitors such as mavacamten, could establish new market openings directed toward cardiomyopathy. Furthermore, it is expected that the increase in the development and approvals, along with the associated biotech company partnerships and ongoing clinical trials, will further fuel growth in the overall treatment of the cardiomyopathy industry. It is the increased prevalence of cardiomyopathy and better-optimized treatment avenues that encourage numerous pharmaceutical companies to invest heavily in various deals relating to the development of these new therapies. If ever these therapies make their way to the market, then the treatment corner would be totally revolutionized for cardiomyopathy patients.
Buy Full Report: https://www.imarcgroup.com/checkout?id=8414&method=809
Marketed Therapies in Cardiomyopathy Market
Camzyos (Mavacamten): Bristol Myers Squibb
Camzyos (mavacamten) is an FDA-approved medication developed by Bristol Myers Squibb for symptomatic obstructive hypertrophic cardiomyopathy (oHCM). As a selective cardiac myosin inhibitor, it works by reducing the overactive contraction of the heart muscle, thereby improving blood flow, and alleviating common symptoms such as shortness of breath and fatigue. Camzyos aims to enhance heart function and quality of life for patients with oHCM, addressing a significant unmet need in the management of this condition.
Tafamidis meglumine: Pfizer
Tafamidis is an approved medication for transthyretin amyloid cardiomyopathy (ATTR-CM), a condition where abnormal protein deposits, called amyloid, accumulate in the heart, and can lead to heart failure. It works by stabilizing the transthyretin (TTR) protein, preventing it from misfolding and forming these harmful deposits. By stabilizing TTR, Tafamidis helps slow the progression of heart-related symptoms and improves heart function in individuals with ATTR-CM. This medication is approved for use in adults with either wild-type or hereditary forms of ATTR-CM.
Emerging Therapies in Cardiomyopathy Market
Aficamten – Cytokinetics
Aficamten, developed by Cytokinetics, is a cardiac myosin inhibitor designed to treat obstructive hypertrophic cardiomyopathy (HCM). Aficamten acts on obstructive hypertrophic cardiomyopathy and helps to modulate selectively decreased contractility of heart muscles. Aficamten could potentially improve the symptoms of, as well as the exercise capacity of, the obstructive HCM patients. Aficamten aims to provide a novel, targeted therapeutic option for those suffering from this debilitating heart condition.
IMB-1018972 (Ninerafaxstat): Imbria Pharmaceuticals
IMB-1018972 (Ninerafaxstat) is an oral modulator of myocardial energetics being developed by Imbria Pharmaceuticals for the treatment of cardiomyopathy. It targets mitochondrial function to optimize energy production in heart cells, aiming to improve cardiac efficiency and reduce symptoms associated with impaired cardiac performance.
Drug Name | Company Name | MOA | ROA |
Aficamten | Cytokinetics | Cardiac myosin inhibitors | Oral |
IMB-1018972 (Ninerafaxstat) | Imbria Pharmaceuticals | Partial fatty acid oxidation inhibitors | Oral |
Detailed list of emerging therapies in Cardiomyopathy is provided in the final report…
Leading Companies in the Cardiomyopathy Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global Cardiomyopathy market, several leading companies are at the forefront of developing integrated platforms to enhance the management of cardiomyopathy. The major players include Bayer, Bristol-Myers Squibb, Pfizer, and others. These companies are driving innovation in the cardiomyopathy market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for cardiomyopathy.
In November 2024, Avidity Biosciences announced its expansion beyond rare skeletal muscle disorders into a new area of focus: precision cardiology. This new initiative aims to target the underlying genetic causes of heart diseases. The company is advancing its first two fully owned precision cardiology candidates: AOC 1086, designed to treat phospholamban (PLN) cardiomyopathy, and AOC 1072, aimed at addressing PRKAG2 Syndrome (Protein Kinase AMP-activated non-catalytic subunit Gamma 2).
In November 2024, Bayer collaborated with Cytokinetics to acquire rights in Japan for the experimental heart drug aficamten. Aficamten targets hypertrophic cardiomyopathy, a genetic condition that impairs heart function. This partnership aims to enhance Bayer’s cardiovascular portfolio and expand treatment options for HCM patients.
Key Players in Cardiomyopathy Market:
The key players in the Cardiomyopathy market who are in different phases of developing different therapies are Avidity Biosciences, Bayer, Cytokinetics, Imbria Pharmaceuticals, Bristol-Myers Squibb, Pfizer, and Others.
Regional Analysis:
The major markets for cardiomyopathy include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for cardiomyopathy while also representing the biggest market for its treatment. Recent advances in cardiomyopathy treatments are giving new hope for better heart health. Gene therapy and stem cell treatment have shown promise in repairing heart tissue and improving heart function. New medication against heart failure is beneficial for the long-term because of its root cause correction. The more effective management of severe cases can also be offered by improved heart transplant techniques and devices such as LVADs. The new inventions will further help to improve survival rates and quality of life in cardiomyopathy patients.
Recent Developments in Cardiomyopathy Market:
· In December 2024, Cytokinetics announced that the U.S. Food & Drug Administration (FDA) has officially accepted its New Drug Application (NDA) for aficamten, a cardiac myosin inhibitor designed to treat obstructive hypertrophic cardiomyopathy (HCM). Under the Prescription Drug User Fee Act (PDUFA), the FDA has assigned the application a standard review process, with a target action date of September 26, 2025. Currently, the FDA does not plan to convene an advisory committee to review the application.
· In December 2024, Cytokinetics confirmed that the European Medicines Agency (EMA) has officially validated its Marketing Authorization Application (MAA) for aficamten. This treatment is aimed at addressing obstructive hypertrophic cardiomyopathy (HCM), a condition that causes thickening of the heart’s walls. This validation begins the process for EMA’s review by the Committee for Medicinal Products for Human Use (CHMP), which will evaluate the potential for aficamten to be approved for use in Europe.
· In September 2024, Bristol Myers Squibb released new long-term data from the EXPLORER-LTE cohort of the MAVA-Long-Term Extension (LTE) study. The findings assessed the efficacy of Camzyos (mavacamten) in adult patients with symptomatic obstructive hypertrophic cardiomyopathy (oHCM) categorized as New York Heart Association (NYHA) class II-III.
· In April 2024, Imbria Pharmaceuticals shared the results of its Phase 2 IMPROVE-HCM clinical trial, which tested ninerafaxstat in patients with symptomatic non-obstructive hypertrophic cardiomyopathy (nHCM). These findings were showcased in a late-breaking clinical trial session at the American College of Cardiology’s Annual Scientific Session & Expo (ACC.24) and subsequently published in the Journal of the American College of Cardiology (JACC).
Key information covered in the report.
Base Year: 2024
Historical Period: 2019-2024
Market Forecast: 2025-2035
Countries Covered
· United States
· Germany
· France
· United Kingdom
· Italy
· Spain
· Japan
Analysis Covered Across Each Country
· Historical, current, and future epidemiology scenario
· Historical, current, and future performance of the cardiomyopathy market
· Historical, current, and future performance of various therapeutic categories in the market
· Sales of various drugs across the cardiomyopathy market
· Reimbursement scenario in the market
· In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current cardiomyopathy marketed drugs and late-stage pipeline drugs.
In-Market Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/cardiomyopathy-market/toc
IMARC Group Offer Other Reports:
Chronic Heart Failure Market: The 7 major chronic heart failure markets reached a value of US$ 6.6 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 18.5 Billion by 2034, exhibiting a growth rate (CAGR) of 9.78% during 2024-2034.
Coronary Heart Disease Market: The 7 major coronary heart disease markets are expected to exhibit a CAGR of 5.17% during 2024-2034.
Cardiac Arrhythmias Market: The 7 major cardiac arrhythmias markets reached a value of US$ 4.9 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 8.0 Billion by 2034, exhibiting a growth rate (CAGR) of 4.57% during 2024-2034.
Myocarditis Market: The 7 major myocarditis markets reached a value of US$ 1,373.4 Million in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 2,281.7 Million by 2034, exhibiting a growth rate (CAGR) of 4.72% during 2024-2034.
Pericarditis Market: The 7 major pericarditis markets reached a value of US$ 2.7 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 4.5 Billion by 2034, exhibiting a growth rate (CAGR) of 4.91% during 2024-2034.
Chronic Obstructive Pulmonary Disease Market: The 7 major chronic obstructive pulmonary disease markets reached a value of US$ 13,464.0 Million in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 18,551.6 Million by 2034, exhibiting a growth rate (CAGR) of 2.96% during 2024-2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.comTel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800