First 100 participants enrolled across Europe and US in largest trial ever performed on glioblastoma patients using ultrasound-based blood-brain barrier opening
PARIS--(BUSINESS WIRE)--Carthera, developer of the revolutionary SonoCloud® medical device, today announces it has enrolled the first 100 patients in its SONOBIRD pivotal trial for the treatment of recurrent glioblastoma. A spin-off of Sorbonne University founded by noted neurosurgeon Prof. Alexandre Carpentier, Carthera reaches this milestone as it prepares to conduct the largest clinical trial ever using ultrasound for the temporary opening of the blood-brain-barrier (BBB) in patients with recurrent glioblastoma.
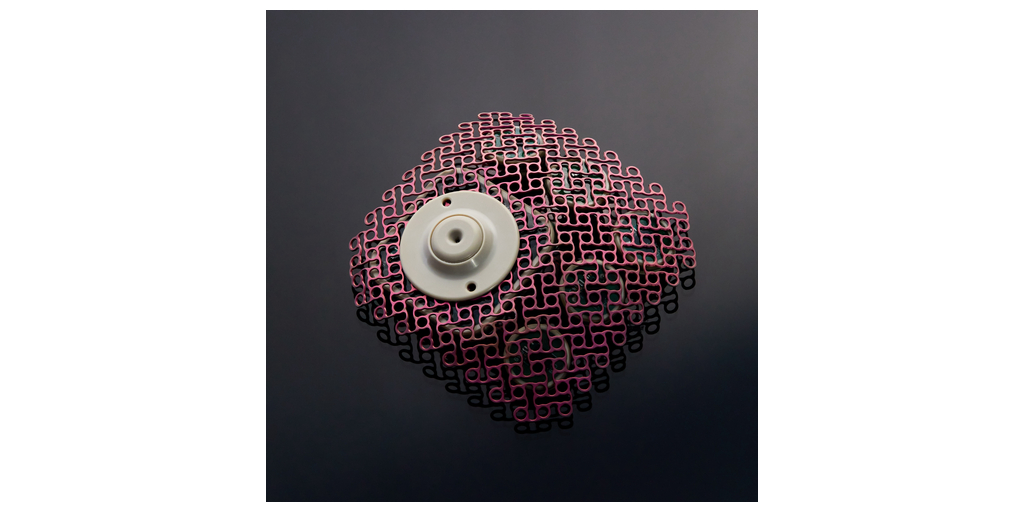


Nearly all 40 participating sites across Europe and the U.S. are now active. Carthera anticipates that recruitment will continue swiftly to reach the 560 patients planned for the study.
The trial (NCT05902169) is the world’s first randomized, multicentric, two-arm pivotal trial of BBB opening prior to chemotherapy injection in recurrent glioblastoma patients. Notably, the trial makes use of the SonoCloud, an innovative ultrasound-based medical device developed by Carthera to treat a wide range of brain disorders. Specifically, the trial compares the use of SonoCloud combined with carboplatin versus standard therapies in patients with a first recurrence of glioblastoma.
“It has been a privilege to offer this trial to patients with recurrent glioblastoma. The underlying concept is highly promising, device implantation has proceeded smoothly and patients have shown strong interest in participating,” said Dr. Brian Gill, assistant professor of neurosurgery at Columbia University Irving Medical Center in New York.
“We desperately need new treatments for patients with recurrent glioblastoma. SonoCloud has the potential to transform our ability to deliver a high dose of therapeutics to the brain,” said Dr. Marjolein Geurts, neuro‐oncologist at Erasmus Medical Center Cancer Institute in Rotterdam, the Netherlands.
To date, more than 550 SonoCloud treatments have been performed worldwide, confirming Carthera’s position as a leader in the field of ultrasound-based BBB opening, as well as the growing interest in SonoCloud as a potentially viable new treatment option for patients with recurrent glioblastoma.
“Our progress in recruiting patients reflects the engagement and strong support from our clinical sites, and from the neuro-oncology and surgery community, in advancing innovative therapies,” said Carole Desseaux, chief clinical officer at Carthera. “We are very grateful for the commitment and enthusiasm of the patients and clinicians who are taking part in this trial.”
“Achieving this milestone is an important step in introducing our SonoCloud device to the large patient population urgently in need of solutions to improve treatment outcomes,” said Frederic Sottilini, CEO of Carthera. “With its breakthrough device and orphan drug designations, Carthera remains committed to transforming glioblastoma treatment and to acquiring market access for its groundbreaking technology.”
Initiated in February 2024, the registrational study aims to enroll a total of 560 US and EU patients within two years, with a view to obtaining marketing authorization. The first interim analysis of the clinical data will be available soon.
About the SONOBIRD trial
The open-label, comparative, randomized, multicenter, two-arm clinical trial with a 1:1 ratio aims to evaluate overall survival in patients undergoing carboplatin chemotherapy and treated with the SonoCloud system to open the Blood-Brain Barrier (BBB). This is compared to the medical consensus recommended regimens (lomustine or temozolomide). The trial also evaluates the effectiveness of the SonoCloud and carboplatin treatment in delaying or slowing tumor growth.
The SONOBIRD trial follows on from the SC9-GBM-01 trial, which demonstrated the feasibility and the safety profile of SonoCloud, as well as the potential of carboplatin tested as a monotherapy in combination with BBB opening.
About SonoCloud
SonoCloud® is an innovative medical device developed by Carthera. It emits ultrasound to temporarily increase the permeability of the blood vessels in the brain to improve the delivery of therapeutic molecules. Invented by Prof. Alexandre Carpentier and developed in collaboration with the Laboratory of Therapeutic Applications of Ultrasound (Laboratoire Thérapie et Applications Ultrasonores, LabTAU, INSERM) in Lyon, France, SonoCloud is an implant inserted into the skull and activated prior to injection of a therapeutic agent. Several minutes of low-intensity ultrasound opens the blood-brain barrier for six hours and increases the concentration of therapeutic molecules in the brain. This ultrasound-induced opening of the blood-brain barrier is a world-first. It offers a new treatment option for a wide range of indications, including brain tumors and Alzheimer’s disease.
SonoCloud is an investigational product, the device has not yet received EMA or FDA approval.
About Carthera
Carthera is a clinical-stage medtech company focused on developing innovative ultrasound-based medical devices to treat a wide range of brain disorders. The company is a spin-off from AP-HP Paris and Sorbonne University. Carthera leverages the inventions of Prof. Alexandre Carpentier, head neurosurgeon at AP-HP Sorbonne university, who has achieved worldwide recognition for his innovative developments in treating brain disorders. Carthera is developing SonoCloud®, an intracranial implant that temporarily opens the blood-brain barrier. The device is currently in clinical trials in Europe and the United States. It received FDA Breakthrough Device Designation in 2022, and FDA/EMA Orphan Drug Designation in 2023 for carboplatin when used with SonoCloud.
Founded in 2010 by Prof. Alexandre Carpentier, run by CEO Frederic Sottilini and chaired by Oern Stuge, MD, Carthera has offices in France (Lyon and Paris) and a subsidiary in Boston, Massachusetts. Since its inception, the technical and clinical development of SonoCloud has received support from the National Research Agency (ANR), the French public investment bank (Bpifrance), the National Institutes of Health (NIH) and the European Innovation Council (EIC). Additional information is available at www.carthera.eu.
Contacts
MEDIA CONTACT
Tyler Coleman
tyler.coleman@wordsatwork.com




