Cerebral Palsy Market Outlook 2025-2035:
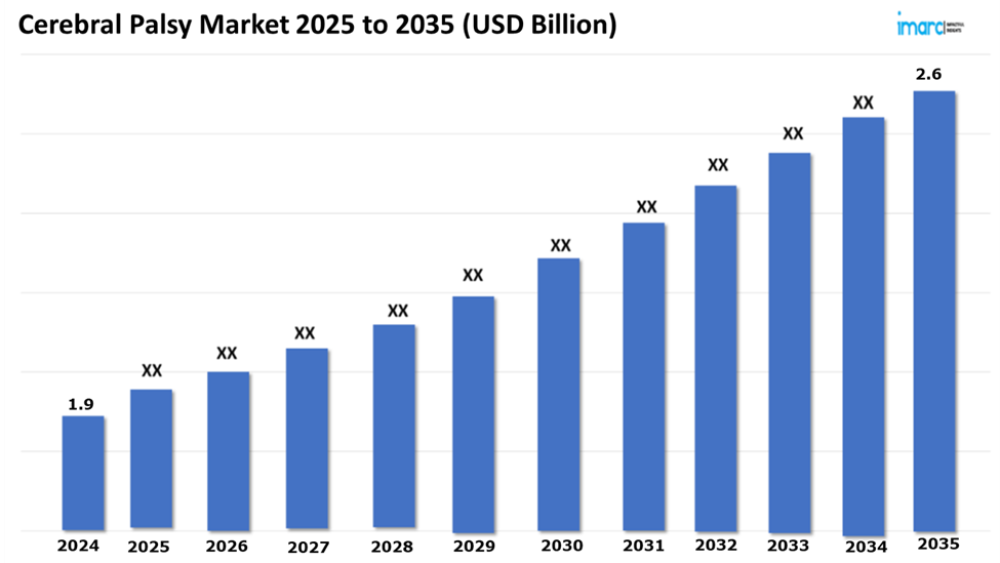
The 7 major cerebral palsy market reached a value of USD 1.9 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 2.6 Billion by 2035, exhibiting a growth rate (CAGR) of 2.59% during 2025-2035. The market for Cerebral Palsy (CP) is fueled by the rapid adoption of noninvasive and minimally invasive treatment options, including botulinum toxin injections, transcranial magnetic stimulation (TMS), and wearable assistive devices. Such advanced therapeutic approaches manage spasticity, enhance motor function, and improve quality of life while reducing the time of recovery and the risks that may result from recovery following an invasive procedure. Innovations such as robotic-assisted therapies, virtual reality-based rehabilitation, and advanced orthotic devices provide targeted interventions against motor impairments, promote neuroplasticity, and improve patient outcomes. Techniques especially appeal to patients and carers seeking efficient, less burdensome, and highly customizable treatment options, contributing to increased patient satisfaction and adherence to therapy.
Advances in Early Detection and Diagnostic Technologies: Driving the Cerebral Palsy Market
Modern diagnostic and treatment technologies, including cerebral palsy, significantly change the management of the disease and outcomes for patients. Modern imaging techniques, like functional MRI (fMRI) and diffusion tensor imaging (DTI), allow the visualization of the details of the brain lesions and nerve pathways, thereby making it accurate for diagnosis and planning with tailored treatment. These advancements are complemented by technologies like motion analysis systems and gait labs that provide clear diagnoses of motor disability so that the clinician can make specific rehabilitation plans. Electrophysiological tests, EMG and EEG are emerging as new technologies to diagnose neuromuscular dysfunctions early intervention. The integration of molecular diagnostics, such as genetic testing and next-generation sequencing, is also gaining importance in establishing the underlying genetic conditions responsible for CP. With artificial intelligence (AI) in imaging and motion analysis, the diagnosis process becomes more accurate as the machine automatically classifies motor impairments, monitors therapy-based progress and minimizes subjective evaluations. These new non-invasive treatments, such as robotic-assisted rehabilitation, transcranial magnetic stimulation (TMS), and advanced wearable exoskeletons, are transformative and help improve motor function, reduce spasticity, and increase independence in patients. Smart wearable devices with sensors enable the real-time monitoring of muscle activity and motor performance, which helps clinicians adjust therapies dynamically and even support at-home rehabilitation programs. These technologies are mainly important in regions where care access is limited and ensure continuous support to patients. Telemedicine platforms are considered a significant factor in widening access to care as they allow remote consultations, virtual therapy sessions, and actual time feedback on rehabilitation exercises. Such platforms ensure that the geographic barrier between patients and healthcare providers can be met timely. These modern advancements together improve the quality of life of individuals with CP, reduce caregiver burden, and promote better long-term outcomes.
Request a PDF Sample Report: https://www.imarcgroup.com/cerebral-palsy-market/requestsample
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The Cerebral Palsy (CP) market is growing immensely with the discovery of recent novel therapies and innovative pharmacological treatments. In view of this, research is being done on recent novel oral and injectable medications targeted at the underlying complex mechanisms involving CP, such as spasticity, dystonia, and neuroinflammation. These innovative treatments have provided greater efficacy, fewer side effects, more accuracy, and a higher level of patient satisfaction in CP management. The field of biological therapies is advancing rapidly, especially for the more severe forms of CP. Recent research addresses chronic neuroinflammation and motor impairments, including the use of monoclonal antibodies that target pro-inflammatory cytokines, such as interleukin-1 and TNF-alpha. These biologics will modulate inflammation at a molecular level to prevent further motor dysfunction and slow disease progression, offering hope for sustained improvements in motor function and quality of life. Innovations in the drug delivery systems, such as liposomal formulation, hydrogels, and nanotechnology-based carriers, are enabling localization and sustained drug delivery to affect sites, such as the spastic muscle or damaged neural pathways. Advanced drug delivery methods deliver higher therapeutic concentrations at the targeted site while maintaining minimal systemic exposure and potential adverse effects, allowing for more effective and safer treatment. Adjunctive therapies, including probiotics and immunomodulators, are under investigation for their ability to enhance neural repair and restore immune system balance, contributing to improved motor outcomes. Combination treatments, which integrate muscle relaxants, anti-inflammatory agents, and neuroprotective compounds, hold significant potential in tackling the complex pathophysiology of CP. Non-invasive pharmacological solutions, such as transdermal patches and advanced topical agents, are also gaining traction due to their simplicity, convenience, and patient-centric design. These approaches promote better adherence to treatment plans while maintaining effectiveness. The ongoing advancements in drug therapies and delivery technologies are transforming the CP treatment landscape, paving the way for improved outcomes and a better quality of life for those affected by the condition.
Buy Full Report: https://www.imarcgroup.com/checkout?id=7687&method=809
Emerging Therapies in Cerebral Palsy Market
UDI-001: Rohto Pharmaceutical
UDI-001 is an investigational therapy developed by Rohto Pharmaceutical, utilizing umbilical cord-derived mesenchymal stromal cells (UC-MSCs) for the treatment of pediatric patients with cerebral palsy (CP) attributed to periventricular leukomalacia (PVL). PVL is a type of white-matter brain injury commonly associated with CP.
Botulinum toxin type A: Hugel
Botulinum toxin type A is a widely used treatment in Cerebral Palsy to manage spasticity and muscle stiffness. By blocking nerve signals to overactive muscles, it reduces spasticity, improves range of motion, and enhances overall motor function. Its minimally invasive nature and targeted action make it a preferred option for improving quality of life in CP patients.
Valbenazine: Neurocrine Biosciences
Valbenazine, a vesicular monoamine transporter 2 (VMAT2) inhibitor, is primarily approved for tardive dyskinesia but has shown potential in managing involuntary movements in Cerebral Palsy, particularly dystonia. Its mechanism reduces excessive dopamine signaling, which may help control abnormal motor activity in CP, though further research is needed to establish its efficacy and safety in this population.
Epoetin alfa biosimilar: LG Chem
Epoetin alfa biosimilar, a recombinant erythropoietin, is being explored in Cerebral Palsy for its potential neuroprotective effects, particularly in reducing inflammation and promoting neuroregeneration. Its role in enhancing oxygen delivery to tissues may also support improved motor function and overall neurological outcomes in CP patients.
AlloRx: Vitro Biopharma
AlloRx, a proprietary allogeneic mesenchymal stem cell (MSC) therapy, is being explored as a potential treatment for Cerebral Palsy. It aims to reduce neuroinflammation, promote neural repair, and improve motor function by leveraging the regenerative and immunomodulatory properties of MSCs, offering hope for improved outcomes in CP patients.
Detailed list of emerging therapies in Cerebral Palsy is provided in the final report…
Leading Companies in the Cerebral Palsy Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global Cerebral Palsy market, several leading companies are at the forefront of developing integrated platforms to enhance the management of Cerebral Palsy. Some of the major players include Neurocrine Bioscience, LG Chem, Vitro Biopharma, and others. These companies are driving innovation in the Cerebral Palsy market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for Cerebral Palsy.
In January 2024, Neurotech International has obtained approval from the Human Research Ethics Committee (HREC) and clearance from the Therapeutic Goods Administration (TGA) to launch a Phase I/II clinical trial of NTI164 for treating cerebral palsy (CP). The trial will assess the safety and effectiveness of NTI164 in pediatric patients with spastic CP, the most common form of the condition.
Key Players in Cerebral Palsy Market:
The key players in the Cerebral Palsy market who are in different phases of developing different therapies are Neurocrine Bioscience, LG Chem, Vitro Biopharma, Hugel, Rohto Pharmaceutical, and Others.
Regional Analysis:
The major markets for Cerebral Palsy include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for Cerebral Palsy while also representing the biggest market for its treatment. Novel treatments for Cerebral Palsy (CP) have recently emerged, including advanced biologics, neuroprotective agents, and precision-targeted therapies, which aim to address the complex underlying mechanisms of the condition. The development of innovative gene therapies and stem cell-based treatment modalities, including allogeneic MSCs, opens the avenues for adaptive solutions to target actual pathologies in CP, such as neuroinflammation, neural damage, and motor dysfunction, with improved outcomes and fewer complications. With recent advancements in the tools and methodologies meant for the detection of CP severity and contributing factors, diagnosis has become earlier and more accurate. Such advanced neuroimaging, motion analysis systems, and AI-powered diagnostics help clinicians devise a personalized treatment strategy with minimal side effects and high efficacy. The other factors propelling the growth of the CP market are increased regulatory approvals, rising investment in research and development (R&D), and growing collaboration between pharmaceutical companies, technology providers, and research institutions. AI-integrated diagnostic platforms and telemedicine solutions are creating opprtunities of high-tech care, especially in less developed regions, thereby ensuring innovative therapies reach a larger patient base. Advanced treatment options and high-tech diagnostic solutions in regions like North America and Europe continue to be at the forefront of innovation, driving the global Cerebral Palsy market toward steady and sustainable growth.
Recent Developments in Cerebral Palsy Market:
· In October 2024, Veezu, the UK’s fastest-growing private hire company, has teamed up with us throughout October to help raise awareness and essential funds. This collaboration is part of Veezu’s ‘Funded by Veezu’ initiative, which strives to make a positive difference in local communities. Veezu’s partnership with Cerebral Palsy Cymru builds on the success of their December 2023 campaign, which raised £10,989 for NSPCC Cymru’s Childline service and funded 2,700 calls to support vulnerable children in need.
· In February 2023, The Cerebral Palsy Research Network has formed a strategic partnership with the CP Alliance Research Foundation (CPARF) to drive research advancements, enhance outcomes, and broaden educational initiatives for the CP community. Through this collaboration, the CP Research Network will work with CPARF to raise funds for CP research, while CPARF will support ongoing research efforts within the network. Additionally, CPARF will help promote our toolkits and webinar series to its community members. The partnership is effective immediately.
Key information covered in the report.
Base Year: 2024
Historical Period: 2019-2024
Market Forecast: 2025-2035
Countries Covered
· United States
· Germany
· France
· United Kingdom
· Italy
· Spain
· Japan
Analysis Covered Across Each Country
· Historical, current, and future epidemiology scenario
· Historical, current, and future performance of the Cerebral Palsy market
· Historical, current, and future performance of various therapeutic categories in the market
· Sales of various drugs across the Cerebral Palsy market
· Reimbursement scenario in the market
· In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current Cerebral Palsy marketed drugs and late-stage pipeline drugs.
In-Market Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/cerebral-palsy-market/toc
IMARC Group Offer Other Reports:
Dry Age Macular Degeneration Market: The 7 major dry age macular degeneration markets are expected to exhibit a CAGR of 4.64% during 2024-2034.
Musculoskeletal Pain Market: The 7 major musculoskeletal pain markets reached a value of US$ 3,975.7 Million in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 5,399.8 Million by 2034, exhibiting a growth rate (CAGR) of 2.82% during 2024-2034.
Cholangiocarcinoma Market: The 7 major Cholangiocarcinoma markets reached a value of US$ 952.5 Million in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 2,590.7 Million by 2034, exhibiting a growth rate (CAGR) of 9.5% during 2024-2034.
Seizures Market: The 7 major seizures markets reached a value of USD 3.2 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 5.0 Billion by 2035, exhibiting a growth rate (CAGR) of 4.03% during 2025-2035.
Alzheimer’s Disease Market: The Alzheimer’s disease market reached a value of US$ 3.1 Billion across the top 7 markets (US, EU4, UK, and Japan) in 2023. Looking forward, IMARC Group expects the top 7 markets to reach US$ 6.3 Billion by 2034, exhibiting a growth rate (CAGR) of 6.8% during 2024-2034.
Dementia Market: The 7 major dementia markets reached a value of US$ 6.8 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 12.8 Billion by 2034, exhibiting a growth rate (CAGR) of 5.85% during 2024-2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.comTel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
The 7 major cerebral palsy market reached a value of USD 1.9 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 2.6 Billion by 2035, exhibiting a growth rate (CAGR) of 2.59% during 2025-2035. The market for Cerebral Palsy (CP) is fueled by the rapid adoption of noninvasive and minimally invasive treatment options, including botulinum toxin injections, transcranial magnetic stimulation (TMS), and wearable assistive devices. Such advanced therapeutic approaches manage spasticity, enhance motor function, and improve quality of life while reducing the time of recovery and the risks that may result from recovery following an invasive procedure. Innovations such as robotic-assisted therapies, virtual reality-based rehabilitation, and advanced orthotic devices provide targeted interventions against motor impairments, promote neuroplasticity, and improve patient outcomes. Techniques especially appeal to patients and carers seeking efficient, less burdensome, and highly customizable treatment options, contributing to increased patient satisfaction and adherence to therapy.
Advances in Early Detection and Diagnostic Technologies: Driving the Cerebral Palsy Market
Modern diagnostic and treatment technologies, including cerebral palsy, significantly change the management of the disease and outcomes for patients. Modern imaging techniques, like functional MRI (fMRI) and diffusion tensor imaging (DTI), allow the visualization of the details of the brain lesions and nerve pathways, thereby making it accurate for diagnosis and planning with tailored treatment. These advancements are complemented by technologies like motion analysis systems and gait labs that provide clear diagnoses of motor disability so that the clinician can make specific rehabilitation plans. Electrophysiological tests, EMG and EEG are emerging as new technologies to diagnose neuromuscular dysfunctions early intervention. The integration of molecular diagnostics, such as genetic testing and next-generation sequencing, is also gaining importance in establishing the underlying genetic conditions responsible for CP. With artificial intelligence (AI) in imaging and motion analysis, the diagnosis process becomes more accurate as the machine automatically classifies motor impairments, monitors therapy-based progress and minimizes subjective evaluations. These new non-invasive treatments, such as robotic-assisted rehabilitation, transcranial magnetic stimulation (TMS), and advanced wearable exoskeletons, are transformative and help improve motor function, reduce spasticity, and increase independence in patients. Smart wearable devices with sensors enable the real-time monitoring of muscle activity and motor performance, which helps clinicians adjust therapies dynamically and even support at-home rehabilitation programs. These technologies are mainly important in regions where care access is limited and ensure continuous support to patients. Telemedicine platforms are considered a significant factor in widening access to care as they allow remote consultations, virtual therapy sessions, and actual time feedback on rehabilitation exercises. Such platforms ensure that the geographic barrier between patients and healthcare providers can be met timely. These modern advancements together improve the quality of life of individuals with CP, reduce caregiver burden, and promote better long-term outcomes.
Request a PDF Sample Report: https://www.imarcgroup.com/cerebral-palsy-market/requestsample
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The Cerebral Palsy (CP) market is growing immensely with the discovery of recent novel therapies and innovative pharmacological treatments. In view of this, research is being done on recent novel oral and injectable medications targeted at the underlying complex mechanisms involving CP, such as spasticity, dystonia, and neuroinflammation. These innovative treatments have provided greater efficacy, fewer side effects, more accuracy, and a higher level of patient satisfaction in CP management. The field of biological therapies is advancing rapidly, especially for the more severe forms of CP. Recent research addresses chronic neuroinflammation and motor impairments, including the use of monoclonal antibodies that target pro-inflammatory cytokines, such as interleukin-1 and TNF-alpha. These biologics will modulate inflammation at a molecular level to prevent further motor dysfunction and slow disease progression, offering hope for sustained improvements in motor function and quality of life. Innovations in the drug delivery systems, such as liposomal formulation, hydrogels, and nanotechnology-based carriers, are enabling localization and sustained drug delivery to affect sites, such as the spastic muscle or damaged neural pathways. Advanced drug delivery methods deliver higher therapeutic concentrations at the targeted site while maintaining minimal systemic exposure and potential adverse effects, allowing for more effective and safer treatment. Adjunctive therapies, including probiotics and immunomodulators, are under investigation for their ability to enhance neural repair and restore immune system balance, contributing to improved motor outcomes. Combination treatments, which integrate muscle relaxants, anti-inflammatory agents, and neuroprotective compounds, hold significant potential in tackling the complex pathophysiology of CP. Non-invasive pharmacological solutions, such as transdermal patches and advanced topical agents, are also gaining traction due to their simplicity, convenience, and patient-centric design. These approaches promote better adherence to treatment plans while maintaining effectiveness. The ongoing advancements in drug therapies and delivery technologies are transforming the CP treatment landscape, paving the way for improved outcomes and a better quality of life for those affected by the condition.
Buy Full Report: https://www.imarcgroup.com/checkout?id=7687&method=809
Emerging Therapies in Cerebral Palsy Market
UDI-001: Rohto Pharmaceutical
UDI-001 is an investigational therapy developed by Rohto Pharmaceutical, utilizing umbilical cord-derived mesenchymal stromal cells (UC-MSCs) for the treatment of pediatric patients with cerebral palsy (CP) attributed to periventricular leukomalacia (PVL). PVL is a type of white-matter brain injury commonly associated with CP.
Botulinum toxin type A: Hugel
Botulinum toxin type A is a widely used treatment in Cerebral Palsy to manage spasticity and muscle stiffness. By blocking nerve signals to overactive muscles, it reduces spasticity, improves range of motion, and enhances overall motor function. Its minimally invasive nature and targeted action make it a preferred option for improving quality of life in CP patients.
Valbenazine: Neurocrine Biosciences
Valbenazine, a vesicular monoamine transporter 2 (VMAT2) inhibitor, is primarily approved for tardive dyskinesia but has shown potential in managing involuntary movements in Cerebral Palsy, particularly dystonia. Its mechanism reduces excessive dopamine signaling, which may help control abnormal motor activity in CP, though further research is needed to establish its efficacy and safety in this population.
Epoetin alfa biosimilar: LG Chem
Epoetin alfa biosimilar, a recombinant erythropoietin, is being explored in Cerebral Palsy for its potential neuroprotective effects, particularly in reducing inflammation and promoting neuroregeneration. Its role in enhancing oxygen delivery to tissues may also support improved motor function and overall neurological outcomes in CP patients.
AlloRx: Vitro Biopharma
AlloRx, a proprietary allogeneic mesenchymal stem cell (MSC) therapy, is being explored as a potential treatment for Cerebral Palsy. It aims to reduce neuroinflammation, promote neural repair, and improve motor function by leveraging the regenerative and immunomodulatory properties of MSCs, offering hope for improved outcomes in CP patients.
| Drug Name | Company Name | MOA | ROA |
| UDI-001 | Rohto Pharmaceutical | Cell replacements | Intravenous |
| Botulinum toxin type A | Hugel | Membrane transport protein modulators; Neuromuscular blocking agents | Intravenous |
| Valbenazine | Neurocrine Biosciences | Vesicular monoamine transporter 2 inhibitors | Oral or via gastrostomy/gastrojejunostomy tube |
| Epoetin alfa biosimilar | LG Chem | Erythropoiesis stimulants; Erythropoietin receptor agonists | Intravenous |
| AlloRx | Vitro Biopharma | Cell replacements | Intravenous Infusion |
Leading Companies in the Cerebral Palsy Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global Cerebral Palsy market, several leading companies are at the forefront of developing integrated platforms to enhance the management of Cerebral Palsy. Some of the major players include Neurocrine Bioscience, LG Chem, Vitro Biopharma, and others. These companies are driving innovation in the Cerebral Palsy market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for Cerebral Palsy.
In January 2024, Neurotech International has obtained approval from the Human Research Ethics Committee (HREC) and clearance from the Therapeutic Goods Administration (TGA) to launch a Phase I/II clinical trial of NTI164 for treating cerebral palsy (CP). The trial will assess the safety and effectiveness of NTI164 in pediatric patients with spastic CP, the most common form of the condition.
Key Players in Cerebral Palsy Market:
The key players in the Cerebral Palsy market who are in different phases of developing different therapies are Neurocrine Bioscience, LG Chem, Vitro Biopharma, Hugel, Rohto Pharmaceutical, and Others.
Regional Analysis:
The major markets for Cerebral Palsy include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for Cerebral Palsy while also representing the biggest market for its treatment. Novel treatments for Cerebral Palsy (CP) have recently emerged, including advanced biologics, neuroprotective agents, and precision-targeted therapies, which aim to address the complex underlying mechanisms of the condition. The development of innovative gene therapies and stem cell-based treatment modalities, including allogeneic MSCs, opens the avenues for adaptive solutions to target actual pathologies in CP, such as neuroinflammation, neural damage, and motor dysfunction, with improved outcomes and fewer complications. With recent advancements in the tools and methodologies meant for the detection of CP severity and contributing factors, diagnosis has become earlier and more accurate. Such advanced neuroimaging, motion analysis systems, and AI-powered diagnostics help clinicians devise a personalized treatment strategy with minimal side effects and high efficacy. The other factors propelling the growth of the CP market are increased regulatory approvals, rising investment in research and development (R&D), and growing collaboration between pharmaceutical companies, technology providers, and research institutions. AI-integrated diagnostic platforms and telemedicine solutions are creating opprtunities of high-tech care, especially in less developed regions, thereby ensuring innovative therapies reach a larger patient base. Advanced treatment options and high-tech diagnostic solutions in regions like North America and Europe continue to be at the forefront of innovation, driving the global Cerebral Palsy market toward steady and sustainable growth.
Recent Developments in Cerebral Palsy Market:
· In October 2024, Veezu, the UK’s fastest-growing private hire company, has teamed up with us throughout October to help raise awareness and essential funds. This collaboration is part of Veezu’s ‘Funded by Veezu’ initiative, which strives to make a positive difference in local communities. Veezu’s partnership with Cerebral Palsy Cymru builds on the success of their December 2023 campaign, which raised £10,989 for NSPCC Cymru’s Childline service and funded 2,700 calls to support vulnerable children in need.
· In February 2023, The Cerebral Palsy Research Network has formed a strategic partnership with the CP Alliance Research Foundation (CPARF) to drive research advancements, enhance outcomes, and broaden educational initiatives for the CP community. Through this collaboration, the CP Research Network will work with CPARF to raise funds for CP research, while CPARF will support ongoing research efforts within the network. Additionally, CPARF will help promote our toolkits and webinar series to its community members. The partnership is effective immediately.
Key information covered in the report.
Base Year: 2024
Historical Period: 2019-2024
Market Forecast: 2025-2035
Countries Covered
· United States
· Germany
· France
· United Kingdom
· Italy
· Spain
· Japan
Analysis Covered Across Each Country
· Historical, current, and future epidemiology scenario
· Historical, current, and future performance of the Cerebral Palsy market
· Historical, current, and future performance of various therapeutic categories in the market
· Sales of various drugs across the Cerebral Palsy market
· Reimbursement scenario in the market
· In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current Cerebral Palsy marketed drugs and late-stage pipeline drugs.
In-Market Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/cerebral-palsy-market/toc
IMARC Group Offer Other Reports:
Dry Age Macular Degeneration Market: The 7 major dry age macular degeneration markets are expected to exhibit a CAGR of 4.64% during 2024-2034.
Musculoskeletal Pain Market: The 7 major musculoskeletal pain markets reached a value of US$ 3,975.7 Million in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 5,399.8 Million by 2034, exhibiting a growth rate (CAGR) of 2.82% during 2024-2034.
Cholangiocarcinoma Market: The 7 major Cholangiocarcinoma markets reached a value of US$ 952.5 Million in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 2,590.7 Million by 2034, exhibiting a growth rate (CAGR) of 9.5% during 2024-2034.
Seizures Market: The 7 major seizures markets reached a value of USD 3.2 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 5.0 Billion by 2035, exhibiting a growth rate (CAGR) of 4.03% during 2025-2035.
Alzheimer’s Disease Market: The Alzheimer’s disease market reached a value of US$ 3.1 Billion across the top 7 markets (US, EU4, UK, and Japan) in 2023. Looking forward, IMARC Group expects the top 7 markets to reach US$ 6.3 Billion by 2034, exhibiting a growth rate (CAGR) of 6.8% during 2024-2034.
Dementia Market: The 7 major dementia markets reached a value of US$ 6.8 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 12.8 Billion by 2034, exhibiting a growth rate (CAGR) of 5.85% during 2024-2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.comTel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800