Chronic Myeloid Leukemia Market Outlook 2025-2035:
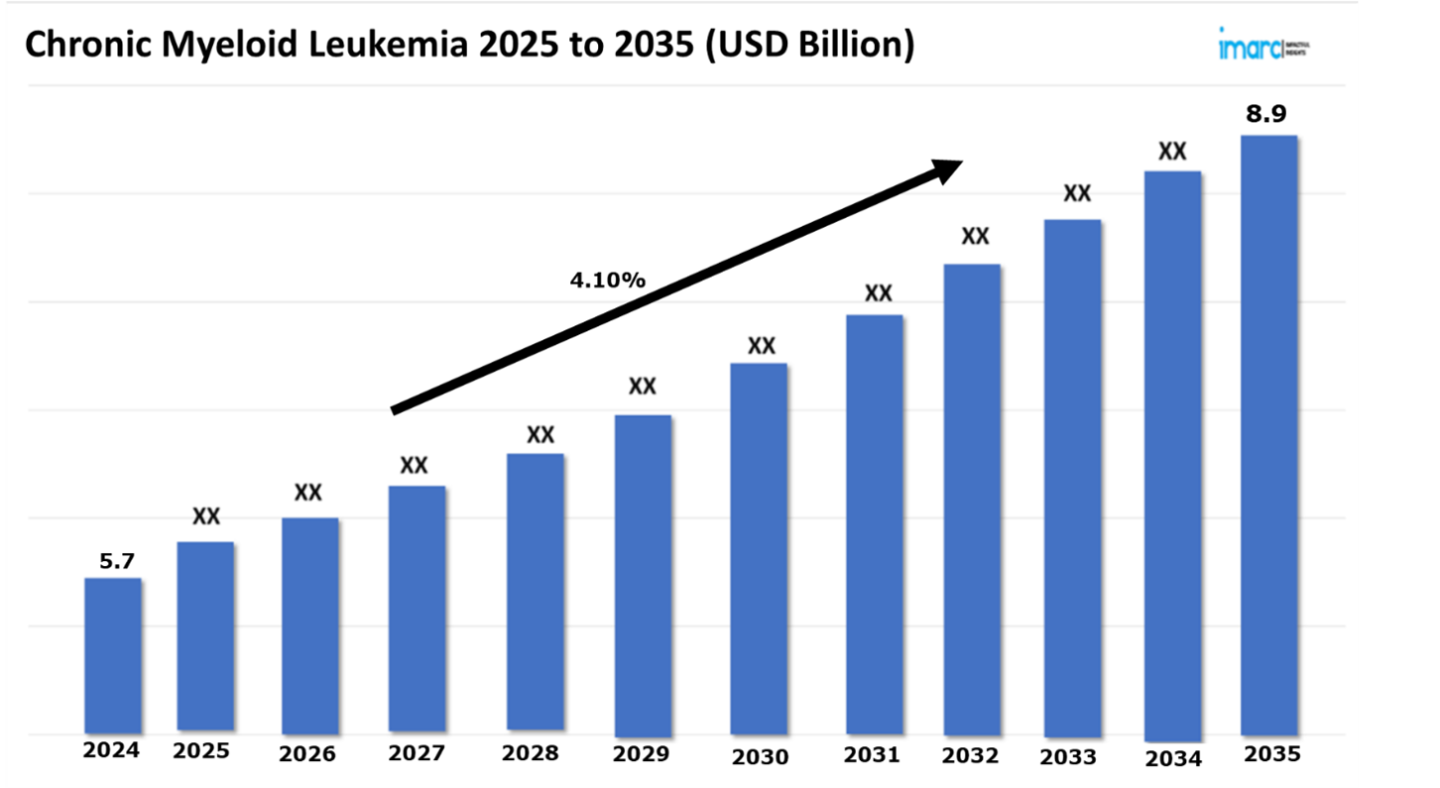
The 7 major chronic myeloid leukemia market reached a value of USD 5.7 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 8.9 Billion by 2035, exhibiting a growth rate (CAGR) of 4.10% during 2025-2035. A fundamental change in chronic myeloid leukemia (CML) management favors precise individual therapeutic approaches instead of conventional general treatments. This evolution stems from a significantly enhanced understanding of the disease’s molecular and genetic roots, most notably the crucial role of the BCR-ABL1 fusion gene in CML development. While established therapies like first-generation tyrosine kinase inhibitors (TKIs) continue to maintain their treatment position yet next-generation TKIs enhance therapy effects through increasing usage or replacing first-generation drugs. These newer agents demonstrate improved efficacy and can overcome resistance mutations, such as the challenging T315I variant. The rise of targeted therapies, encompassing allosteric inhibitors and innovative combination regimens, provide targeted treatments that minimize adverse effects and boost patient long-term success. These novel approaches are especially beneficial for those who have developed resistance or intolerance to conventional TKIs. Furthermore, combination therapies that target multiple disease pathways are showing promise in tackling refractory or advanced-phase CML cases, thus expanding the therapeutic options available for complex and challenging scenarios.
Revolutionizing Chronic Myeloid Leukemia Treatment: The Impact of Targeted Therapy
Medical advances in targeted therapy treatment produced revolutionary changes to Chronic Myeloid Leukemia (CML) which delivered improved results for patients. The core transformation of this evolution relies on developing three tyrosine kinase inhibitors (TKIs) called imatinib dasatinib and nilotinib. The BCR-ABL1 fusion protein leads to CML, and these new drug treatments direct their action exclusively against this critical cause. This precise action allows for effective disease control with significantly reduced side effects, transforming CML from a life-threatening illness into a manageable chronic condition for many. In addition, the availability of newer generation tyrosine kinase inhibitors bosutinib and ponatinib delivers essential treatment choices for patients who no longer respond or tolerate their original therapies. Such drugs display improved resistance mechanisms while effectively targeting challenging T315I mutations and demonstrate minimized side effects for better long-term treatment endurance. The move towards personalized medicine, facilitated by genetic testing, is also playing a key role, allowing for individualized TKI selection and further optimizing treatment plans. These advancements collectively contribute not only to improved survival rates but also to enhancing the quality of life for individuals living with CML, fuelling ongoing progress and innovation within the market
Request a PDF Sample Report:
https://www.imarcgroup.com/chronic-myeloid-leukemia-market/requestsample
Transforming Chronic Myeloid Leukemia Management: Advances in Targeted Therapies and Innovative Approaches
The therapeutic landscape for chronic myeloid leukemia treatment continues its remarkable evolution through innovative therapies together with treatment methods refinement. The foundation of new treatment advancements centers on the BCR-ABL1 fusion protein which serves as the major molecular agent responsible for driving CML. The introduction of tyrosine kinase inhibitors (TKIs), like imatinib, dasatinib, and nilotinib, marked a paradigm shift, allowing for effective disease control, minimized side effects, and enhanced patient well-being. Furthermore, the development of newer-generation TKIs, such as bosutinib and ponatinib, has broadened the therapeutic arsenal, offering crucial alternatives for patients who develop resistance or intolerance to initial treatments. Notably, these advanced TKIs directly target difficult-to-manage mutations, including T315I, which had previously posed a significant obstacle. The current trajectory emphasizes combination therapies and the pursuit of treatment-free remission (TFR) strategies, pushing the boundaries of optimized care for both newly diagnosed and challenging refractory cases. The horizon of CML treatment also includes the promise of gene-based therapies and novel inhibitors, potentially overcoming resistance mechanisms and facilitating deeper molecular responses. These collective strides are revolutionizing CML care, providing more tailored and impactful solutions, and fostering renewed hope for improved survival and quality of life for patients.
Buy Full Report:
https://www.imarcgroup.com/checkout?id=7958&method=809
Marketed Therapies in the Chronic Myeloid Leukemia Market
Sprycel (Dasatinib) - Bristol-Myers Squibb
Sprycel (dasatinib), developed by Bristol-Myers Squibb, is a second-generation tyrosine kinase inhibitor (TKI) designed to treat Chronic Myeloid Leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL).
Tasigna (Nilotinib) - Novartis
Tasigna (nilotinib), developed by Novartis, is a second-generation tyrosine kinase inhibitor (TKI) specifically designed for the treatment of Chronic Myeloid Leukemia (CML) in adults with Philadelphia chromosome-positive (Ph+) disease. Approved by the FDA in 2007, Tasigna is indicated for newly diagnosed CML patients in the chronic phase, as well as for those who are resistant or intolerant to prior therapies, including imatinib.
Emerging Therapies in the Chronic Myeloid Leukemia Market
Navtemadlin - Kartos Therapeutics
Navtemadlin (KRT-232), currently under investigation, represents a compelling new approach in the treatment of Chronic Myeloid Leukemia (CML). This small-molecule inhibitor is designed to specifically target MDM2, a protein crucial in the regulation of the tumor suppressor p53.
ELVN-001 - Enliven Therapeutics
ELVN-001, an investigational therapy under development by Elevian, offers a promising new direction in the treatment of Chronic Myeloid Leukemia (CML). This novel approach seeks to overcome limitations presented by currently available tyrosine kinase inhibitors (TKIs), the mainstay of CML treatment.
AOP2014 - AOP Orphan Pharmaceuticals/PharmaEssentia Corporation
AOP2014, an investigational therapy developed by AOP Orphan Pharmaceuticals, is an emerging treatment for Chronic Myeloid Leukemia (CML). This novel compound belongs to a new class of targeted therapies aimed at addressing specific mutations and overcoming resistance in CML patients, especially those with limited treatment options due to resistance to first- and second-generation tyrosine kinase inhibitors (TKIs).
Drug Name | Company Name | MOA | ROA |
Navtemadlin | Kartos Therapeutics | Proto-oncogene protein c-mdm2 inhibitors | Navtemadlin |
ELVN-001 | Enliven Therapeutics | Protein tyrosine kinase inhibitors | Oral |
AOP2014 | AOP Orphan Pharmaceuticals/PharmaEssentia Corporation | Interferon alpha-2 replacements | Subcutaneous |
Detailed list of emerging therapies in Chronic Myeloid Leukemia is provided in the final report…
Leading Companies in the Chronic Myeloid Leukemia Market:
The Chronic Myeloid Leukemia (CML) treatment landscape is undergoing a period of rapid transformation, fuelled by relentless innovation and substantial investment from major pharmaceutical players. Companies like Novartis, Bristol-Myers Squibb, Kartos Therapeutics, Enliven Therapeutics, AOP Orphan Pharmaceuticals/PharmaEssentia Corporation are at the vanguard, actively pursuing the development of cutting-edge therapies to tackle the complexities of CML management. Their research pipeline is rich and varied, moving beyond established first-generation tyrosine kinase inhibitors (TKIs) to include second-generation options like dasatinib and nilotinib, while also pushing boundaries with newer agents such as ponatinib and bosutinib that specifically target resistance mutations. Furthermore, investigating combination therapies, gene-based approaches, and allosteric inhibitors reflects a concerted effort to overcome treatment resistance and enhance outcomes for patients battling advanced or refractory CML. These advancements are not replacing but rather complementing the existing TKI foundation, with a growing focus on precision medicine and personalized treatment strategies. The strategic adoption of TKI therapy in earlier disease phases and the ambitious pursuit of treatment-free remission (TFR) for select patients highlight the industry’s commitment to tailoring treatment to individual patient needs. This continuous drive for innovation and targeted therapeutics is ultimately aimed at delivering safer, more effective, and personalized treatment options, leading to improved survival rates and enhanced quality of life for CML patients.
In October 2024, the Phase 1 clinical trial ELVN-001 showcased promising results in a challenging patient population: individuals with Chronic Myeloid Leukemia (CML) who had become resistant to, refractory to, or intolerant of tyrosine kinase inhibitors (TKIs), the standard first-line therapy for this disease.
Key Players in the Chronic Myeloid Leukemia Market:
The key players in Chronic Myeloid Leukemia who are in different phases of developing different therapies are Novartis, Bristol-Myers Squibb, Kartos Therapeutics, Enliven Therapeutics, AOP Orphan Pharmaceuticals/PharmaEssentia Corporation, and others.
Regional Analysis:
The Chronic Myeloid Leukemia (CML) treatment market is witnessing remarkable growth, propelled by innovative therapies and a significant move towards personalized medicine. Leading healthcare markets such as the United States, Europe, and Japan are spearheading this expansion, with the US holding a prominent position due to its substantial patient base and robust research capabilities. This market surge is primarily fuelled by the abandonment of older, less targeted therapies like chemotherapy and interferon, in favor of sophisticated tyrosine kinase inhibitors (TKIs) and other emerging treatments. These groundbreaking advancements zero in on the specific genetic and molecular underpinnings of CML, most notably the BCR-ABL1 fusion protein, resulting in higher efficacy and fewer side effects than their predecessors. The field of CML management is undergoing a fundamental shift, moving away from blanket treatments to individualized, targeted approaches. While TKIs such as imatinib, dasatinib, and nilotinib have established themselves as cornerstones of treatment, newer generations of TKIs like ponatinib and bosutinib provide crucial options for patients who develop resistance or intolerance to first-line drugs. Furthermore, the future of CML treatment is being shaped by the investigation of gene-based therapies, allosteric inhibitors, and combination regimens, offering new possibilities for patients with resistant or advanced disease.
Recent Developments in the Chronic Myeloid Leukemia Market:
· In November 2024, The U.S. Food and Drug Administration (FDA) has granted accelerated approval for asciminib (Scemblix) as a treatment for adults with newly diagnosed Philadelphia chromosome (Ph)-positive Chronic Myeloid Leukemia (CML) in the chronic phase.
· In October 2024, the Phase 1 clinical trial ELVN-001 showcased promising results in a challenging patient population: individuals with Chronic Myeloid Leukemia (CML) who had become resistant to, refractory to, or intolerant of tyrosine kinase inhibitors (TKIs), the standard first-line therapy for this disease.
Key information covered in the report.
Base Year: 2024
Historical Period: 2019-2024
Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the Chronic Myeloid Leukemia market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the Chronic Myeloid Leukemia market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current Chronic Myeloid Leukemia marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC & List of Figures:
https://www.imarcgroup.com/chronic-myeloid-leukemia-market/toc
IMARC Group Offer Other Reports:
Diffuse Large B-cell Lymphoma (DLBCL) Market: The 7 major Diffuse- large B-cell lymphoma market reached a value of USD 4,013.3 Million in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 5,405.9 Million by 2035, exhibiting a growth rate (CAGR) of 2.8% during 2025-2035.
Non-Hodgkin’s Lymphoma Market: The 7 major Non-Hodgkins lymphoma market reached a value of US$ 4.0 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 7.4 Billion by 2034, exhibiting a growth rate (CAGR) of 5.71% during 2024-2034.
Follicular Lymphoma Market: The 7 major Follicular Lymphoma market reached a value of US$ 1.6 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 2.2 Billion by 2034, exhibiting a growth rate (CAGR) of 3.16% during 2024-2034.
Inflammatory Bowel Disease Market: The 7 major inflammatory bowel disease markets reached a value of USD 14.92 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 20.08 Billion by 2035, exhibiting a growth rate (CAGR) of 2.73% during 2025-2035.
Cancer Cachexia Market: The 7 major cancer cachexia markets are expected to exhibit a CAGR of 3.77% during 2024-2034.
Cutaneous T-Cell Lymphoma Market: The 7 major Cutaneous T-cell-lymphoma market reached a value of US$ 428.2 Million in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 628.7 Million by 2034, exhibiting a growth rate (CAGR) of 3.55% during 2024-2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800