Chronic Urticaria Market Outlook 2025-2035:
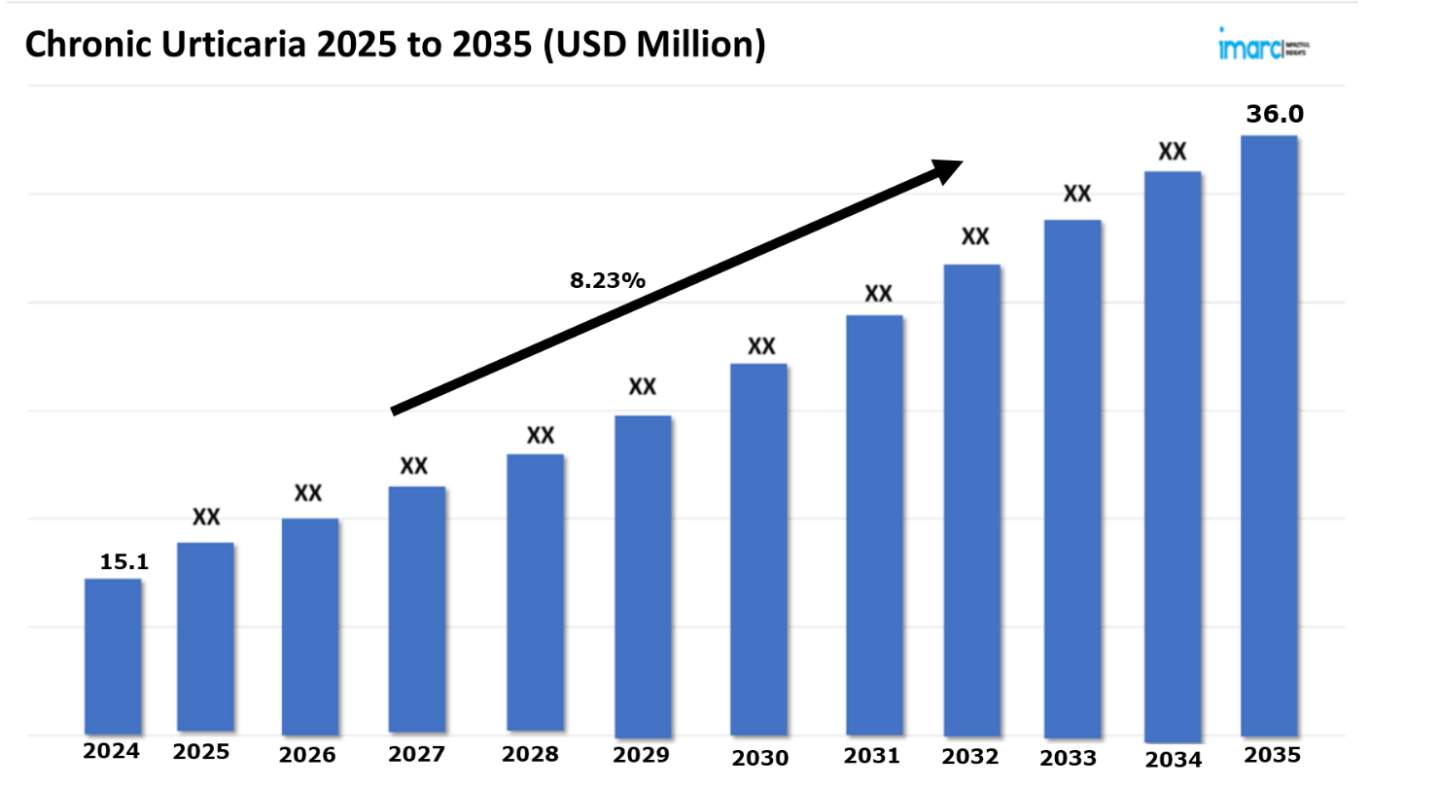
The 7 major chronic urticaria market reached a value of USD 15.1 Million in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 36.0 Million by 2035, exhibiting a growth rate (CAGR) of 8.23% during 2025-2035. The industry of chronic urticaria (CU) management is currently witnessing a significant transformation , gravitating away from generalized, conventional treatments toward a more customized and accurate approach. This inclination is mainly influenced by a magnifying understanding of the complicated mechanisms underlying CU, encompassing the chief exhibited by intricate inflammatory pathways, autoimmune responses, and mast cell activation. While corticosteroids and antihistamines remain core in numerous treatment methodologies, there’s an intensifying trend to substitute or aid them with targeted biologic therapies. Omalizumab (Xolair), for instance, has showcased substantial success in managing symptoms, especially in patients who depict limited response to traditional treatments, by directly targeting particular elements of the immune system.
Revolutionizing Chronic Urticaria Treatment: The Impact of Targeted Therapy
The treatment of chronic urticaria (CU), a condition highlighted by constant itching and hives, is undergoing a notable breakthrough, which majorly pertains to the emergence of targeted therapies. The time when corticosteroids and antihistamines served as the chief, yet often insufficient, treatment choices has passed. Now, precision medicine and biologics are at the forefront, providing hope for individuals who have been navigating treatment options for relief. The emergence of monoclonal antibodies such as omalizumab (Xolair), which particularly targets IgE, highlights a revolutionary shift. This therapy has established itself as a highly efficient method in managing symptoms for those with moderate to severe CU, offering not only a mitigation for hives but also a reduction in the debilitating side effects generally related to the conventional treatments. Such targeted therapies reflect an inclination towards catering to the core immune mechanisms influencing the disease, providing a more efficient and accurate solution. Further diversifying the options for CU management are newer treatments like JAK inhibitors and other immunomodulatory agents. These advancements are particularly crucial for patients with CU that is resistant to conventional therapies or has become chronic. By providing enhanced effectiveness and a better accurate approach to mitigating and dealing with the underlying immune dysfunction, such treatment exhibits prospects not just for symptom management, but also greater long-term outcomes. Notably, such newer and innovative agents can target several pathways involved in the complicated pathogenesis of CU, providing an extensive approach to disease control that was previously unfeasible. The revolutionizing dynamics of targeted therapies offers a more intricated and promising path forward for patients suffering with the often-debilitating effects of chronic urticaria.
Request a PDF Sample Report:
https://www.imarcgroup.com/chronic-urticaria-market/requestsample
Transforming Chronic Urticaria Treatment: The Impact of Personalized Medicine
The burgeoning field of personalized medicine is revolutionizing how Chronic Urticaria (CU) is approached, shifting away from a one-size-fits-all model toward highly tailored treatment strategies. This approach hinges on the ability to delve into the unique immune landscape of each patient, identifying the specific mechanisms driving their urticaria. By pinpointing these individual triggers, healthcare providers can construct treatment plans that directly address the root causes of the condition, leading to more effective symptom control and a quicker path to relief. Instead of relying on a trial-and-error method often associated with traditional treatments, personalized medicine facilitates the selection of the most appropriate therapies upfront, minimizing exposure to ineffective or potentially harmful medications. This precision not only leads to improved patient outcomes and reduced side effects but also optimizes resource allocation within the healthcare system, reducing the burden of unnecessary treatments. Furthermore, when patients receive treatments that are aligned with their specific condition, they experience greater satisfaction and are more likely to adhere to their prescribed regimen over the long term, a crucial factor in the sustained management of chronic conditions like CU. In essence, personalized medicine is not just enhancing the efficacy of CU treatments; it is enhancing the overall quality of life for those living with this challenging condition.
Buy Full Report:
https://www.imarcgroup.com/checkout?id=6725&method=809
Marketed Therapies in the Chronic Urticaria Market
Fexofenadine – Sanofi
Fexofenadine, marketed under the brand name Allegra by Sanofi, is a second-generation antihistamine commonly used to treat allergic conditions, including Chronic Urticaria (CU). It works by selectively blocking H1 histamine receptors, thereby preventing histamine from causing allergic symptoms such as itching, swelling, and hives that are characteristic of CU.
Desloratadine - Merck & Co
Desloratadine, a prominent second-generation antihistamine, is frequently prescribed under the brand name Clarinex by Merck & Co. Its widespread use stems from its effectiveness in managing Chronic Urticaria (CU), a condition characterized by persistent and often debilitating hives.
Xolair (omalizumab) – Roche/Novartis
Xolair (omalizumab), developed by Roche and Novartis, is a monoclonal antibody that has emerged as a groundbreaking treatment for Chronic Urticaria (CU), particularly for patients with chronic spontaneous urticaria (CSU) that is resistant to standard therapies such as antihistamines and corticosteroids.
Emerging Therapies in the Chronic Urticaria Market
Rilzabrutinib - Sanofi
Rilzabrutinib, an investigational therapeutic agent developed by Principia Biopharma (now a subsidiary of Sanofi), represents a promising approach in the treatment of chronic urticaria (CU). This particular molecule is classified as a Bruton’s tyrosine kinase (BTK) inhibitor, a class of drugs known for their role in regulating immune cell function.
Remibrutinib – Novartis
Remibrutinib, developed by Novartis, is an investigational Bruton’s tyrosine kinase (BTK) inhibitor being explored for the treatment of Chronic Urticaria (CU), particularly chronic spontaneous urticaria (CSU). BTK is an enzyme critical in the signalling pathways of immune cells such as mast cells, basophils, and B cells, which are involved in the inflammatory processes underlying urticaria.
Drug Name | Company Name | MOA | ROA |
Rilzabrutinib | Sanofi | Agammaglobulinaemia tyrosine kinase inhibitors | Oral |
Remibrutinib | Novartis | Agammaglobulinaemia tyrosine kinase inhibitors | Oral |
Detailed list of emerging therapies in Chronic Urticaria is provided in the final report…
Leading Companies in the Chronic Urticaria Market:
The landscape of Chronic Urticaria (CU) treatment is currently experiencing a notable period of innovation and progress, largely due to the extensive research and development initiatives undertaken by several leading pharmaceutical companies. Among these industry frontrunners, Novartis, Roche, Sanofi, and Merck & Co are making substantial strides in the creation of cutting-edge therapies designed to enhance symptom management and improve long-term outcomes for CU patients. Furthermore, the examination of JAK inhibitors, BTK inhibitors, and immune-modulating agents demonstrates a collective effort to target the underlying immune mechanisms responsible for CU. This approach promises to yield more precise and effective treatments for patients grappling with severe or treatment-resistant forms of the disease. It is important to note that these advances are intended to supplement and improve existing treatments, rather than replace them entirely. As the industry moves towards personalized treatment strategies, the emphasis on tailoring therapies to individual patients’ immune profiles and responses becomes increasingly critical. This shift is indicative of the broader trend towards precision medicine, which holds the potential to revolutionize CU management.
In February 2024, Sanofi’s experimental drug, rilzabrutinib, has shown promising results in a Phase II clinical trial for patients with chronic spontaneous urticaria, a condition marked by persistent hives and intense itching.
Key Players in the Chronic Urticaria Market:
The key players in Chronic Urticaria who are in different phases of developing different therapies are Novartis, Roche, Sanofi, Merck & Co., and others.
Regional Analysis:
The Chronic Urticaria (CU) treatment market is experiencing significant growth, driven by innovative therapies and a strong shift towards personalized medicine. Key healthcare markets, including the United States, Europe, and Japan, are at the forefront of this expansion, with the US leading due to its large patient population and advanced research infrastructure. This surge is primarily driven by the move away from traditional treatments like antihistamines and corticosteroids, toward more targeted biologic therapies and immune-modulating treatments. These advancements specifically target the immune pathways involved in chronic urticaria, improving efficacy and minimizing side effects compared to older therapies. The management of CU is undergoing a profound transformation, focusing on individualized treatment approaches. While therapies like omalizumab (Xolair) have become foundational in treating chronic spontaneous urticaria (CSU), newer treatments, such as dupilumab (Dupixent) and JAK inhibitors, provide crucial options for patients with severe or refractory disease. Additionally, the future of CU management is being shaped by the exploration of combination therapies, gene-based treatments, and novel immune modulators, offering exciting new possibilities for patients who do not respond to conventional treatments.
Recent Developments in the Chronic Urticaria Market:
· In February 2024, Sanofi’s experimental drug, rilzabrutinib, has shown promising results in a Phase II clinical trial for patients with chronic urticaria, a condition marked by persistent hives and intense itching.
· In May 2024, the culmination of a 52-week trial provided compelling evidence for the favourable safety profile of remibrutinib, a promising pharmaceutical compound. This extended study period is crucial, as it allows for a thorough assessment of a drug’s long-term tolerability.
Key information covered in the report.
Base Year: 2024
Historical Period: 2019-2024
Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the Chronic Urticaria market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the Chronic Urticaria market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current Chronic Urticaria marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC & List of Figures:
https://www.imarcgroup.com/chronic-urticaria-market/toc
IMARC Group Offer Other Reports:
Acute Intermittent Porphyria Market: The 7 major acute intermittent porphyria markets reached a value of US$ 1.7 Million in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 2.4 Million by 2034, exhibiting a growth rate (CAGR) of 3.46% during 2024-2034.
Acute Pain Market: The 7 major acute pain markets reached a value of USD 83.5 Million in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 129.8 Million by 2035, exhibiting a growth rate (CAGR) of 4.09% during 2025-2035.
Sepsis Market: The 7 major Sepsis market reached a value of US$ 438.4 Million in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 907.3 Million by 2034, exhibiting a growth rate (CAGR) of 6.84% during 2024-2034
Venous Thromboembolism Market: The 7 major venous thromboembolism markets reached a value of US$ 3.8 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 7.0 Billion by 2034, exhibiting a growth rate (CAGR) of 5.71% during 2024-2034.
Metabolic Acidosis Market: The 7 major Metabolic acidosis market are expected to exhibit a CAGR of 5.76% during 2024-2034.
Angioedema Market: The 7 major Angioedema market are expected to exhibit a CAGR of 7.01% during 2025-2035.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800