MURRIETA, Calif., Dec. 6, 2024 /PRNewswire/ -- Copan Diagnostics, a leader in microbiology laboratory innovations, is proud to announce that UriSponge®, a urine collection and transport device which uses a new formulation of advanced preservatives to ensure specimen stability for culture, has received clearance from the U.S. Food and Drug Administration (FDA). The solution offers clinical laboratories and healthcare providers a user-friendly, reliable, and cost-effective way to improve urine specimen collection, preservation, and transport.
Revolutionizing Urine Collection
"Copan is dedicated to advancing laboratory practices through groundbreaking innovation," said Fabrizio Mazzochi, CEO of Copan Diagnostics. "UriSponge® provides a reliable and efficient solution that supports laboratory needs while offering healthcare providers a safe, streamlined process."
Unique System for Urine Specimen Preservation
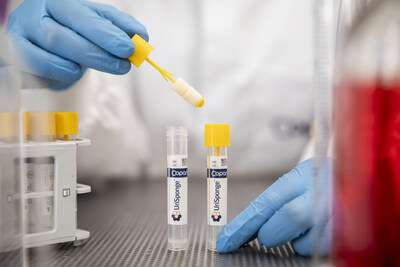
Urine samples constitute nearly 70% of specimens processed annually in clinical laboratories. Urine specimens are collected and processed to cultivate uropathogenic bacteria and yeasts. UriSponge® enables urine transfer from sterile container to tube using a quick dip-and-close method reducing steps and improving workflow.
The system features a screw-cap tube with a plastic applicator and sponges loaded with preservatives. Upon contact with urine, these preservatives maintain sample stability during transport, providing dependable results and minimizing the risk of contamination or degradation. The unique design ensures accurate sample preservation without requiring precise fill lines or additional mixing, reducing complexity and the use of extra consumables.
UriSponge® is designed with advanced preservatives to ensure specimen integrity, offering an alternative to traditional methods that require stringent handling protocols.
Automation Compatible
UriSponge® integrates seamlessly with laboratory automation such as Copan's WASP® Walk-Away Specimen Processor, which automates essential microbiology specimen processing tasks, including planting and streaking. UriSponge® and Copan's full laboratory automation solution offer a streamlined approach to specimen handling from collection through analysis, aimed at enhancing laboratory productivity and consistency.
Already available worldwide, Copan's UriSponge® will be accessible through U.S. distribution partners early in 2025.
About Copan Diagnostics, Inc.
Copan Diagnostics is part of Copan Group, a leading global manufacturer of collection and transport systems. Through its collaborative approach, Copan has developed breakthrough technologies such as FLOQSwabs®, ESwab®, UTM® Universal Transport Medium™, and Full Laboratory Automation and artificial intelligence. Copan continues to innovate and transform Collection and Transport systems, as well as laboratory automation, helping healthcare providers improve healthcare patient outcomes.
For more information, visit the copanusa.com.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/copan-diagnostics-announces-fda-clearance-for-innovative-urine-collection-device-302324563.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/copan-diagnostics-announces-fda-clearance-for-innovative-urine-collection-device-302324563.html
SOURCE Copan Diagnostics, Inc.