- Presentation by CSO and co-founder Dr. Yifan Song to include data demonstrating Cyrus’s hybrid AI/screening platform in risk mitigation of protein therapeutics.
- Cyrus IgG protease candidate has verified extended half-life and reduced immunogenicity with demonstrated repeat dosing in vivo.
- Cyrus’s technology aims to develop protein therapeutics with best-in-class properties
SEATTLE--(BUSINESS WIRE)--Cyrus Biotechnology, Inc., a Seattle-based biotechnology company with a novel hybrid AI/screening platform for biologics discovery, will present the company’s capabilities in protein engineering and therapeutics risk mitigation at the inaugural Hit ID Summit in Boston, Tuesday November 12 at 1pm at the Boston Back Bay Hilton. The presentation by Dr. Yifan Song, Chief Science Officer (CSO) and Cyrus Co-founder will also include preclinical work validating a next generation IgG-degrader (Immunoglobulin degrading enzyme from S. pyogenes, IdeS) for autoimmune disease and gene therapy pre-treatment. The IdeS program demonstrates the utility of Cyrus’s protein engineering platform for optimizing biologics to build validated, derisked, and readily manufacturable development candidates.
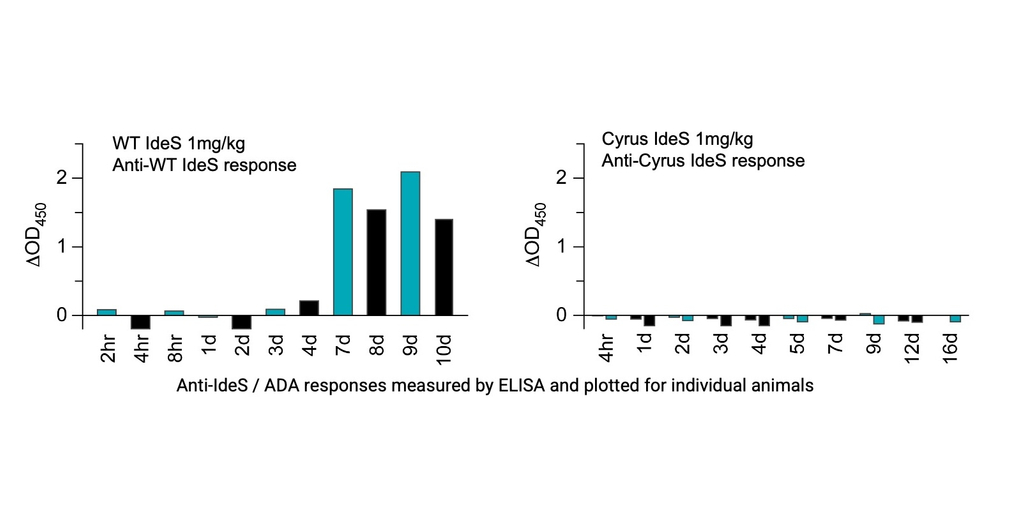


In the presentation, Dr. Song will demonstrate Cyrus’s capabilities in developing a best-in-class IdeS using in silico AI, protein design algorithms from the 2024 Nobel Prize winning Baker Lab, and high-throughput laboratory screening. These have been used by Cyrus for dozens of collaborations including with Genentech, Janssen, and Selecta Bio. Cyrus’s IdeS candidate has been optimized for all the key therapeutic features, including serum half-life, enzymatic activity, stability, and B cell and T cell immunogenicity.
The presentation will show Cyrus’s IdeS candidate has substantially extended half-life and pharmacodynamics compared to the wild type IdeS, rapidly reducing serum IgG level and maintaining low IgG titers for at least 14 days in rabbits, compared to a duration of less than 7 days for the wild type.
Cyrus’s candidate which demonstrates near-wild-type IgG degrading activity in vitro and in vivo, can be titrated to achieve different degrees of IgG reduction, and is effective at very low doses.
Cyrus employs a proprietary platform for reducing the immunogenicity of protein therapeutics. The approved WT IdeS enzyme therapeutic elicits a very strong anti-IdeS antibody response upon first dose and is not redosable in the clinic. Cyrus has substantially reduced the binding of pre-existing anti-IdeS antibodies - by orders of magnitude in one assay format - and even in human sera with high anti-IdeS antibody titers.
Novel data generated in rabbits exhibits that Cyrus's reduced immunogenicity IdeS does not elicit anti-IdeS antibodies for a minimum of two weeks after administration, while wild type IdeS induces strong anti-IdeS antibodies under the same conditions (see figure below). In a multi-dose study, low doses of Cyrus’s IdeS retain strong activity upon repeat dosing while the WT IdeS loses efficacy after a first dose.
“These newly generated data, combined with titratable potency at very low doses, will permit Cyrus's IdeS to be administered via convenient subcutaneous injection at a frequency suitable for chronic indications such as autoimmune Immune Thrombocytopenia (ITP) and generalized Myasthenia Gravis (gMG),” said Lucas Nivon, Cyrus Biotechnology CEO and Co-founder. “The efficacy of IgG reduction has been shown by the FcRn inhibitor class in the clinic for gMG. Cyrus’s IdeS has more rapid IgG reduction, better IgG-lowering potency, and likely more convenient administration than the anti-FcRn class, with the potential for best-in-disease performance.”
Hit ID Summit
The Hit ID Summit provides the ideal environment for medicinal chemistry, computational chemists & drug discovery experts from both large pharma and biotechs to unite, share experiences & reach conclusions to be applied in hit screening strategies.
About Cyrus Biotechnology
Cyrus Biotechnology is a pre-clinical-stage AI-driven therapeutics company with an internal pipeline of novel biologics primarily in autoimmune indications. Cyrus is advancing a next generation IdeS IgG protease with half-life extension and immunogenicity optimization, for treatment of IgG-mediated autoimmune indications, into IND-enabling studies, as well as advancing multiple discovery stage cytokine programs. Cyrus was co-founded with Prof. David Baker, 2024 Nobel Laureate in Chemistry, inventor of Rosetta and numerous protein design AI tools, and has worked with dozens of Pharma firms on protein redesign for novel therapeutics, including Genentech and Janssen, and has contributed to cytokine optimization with half a dozen partners. In 2021 Cyrus started to develop its own therapeutics.
For more information about Cyrus please visit https://cyrusbio.com/
NOTICE: The information contained in this document is dated as of November 11, 2024. Cyrus Biotechnology, Inc. (the Company) disclaims any obligation to update such information after such date. This document contains forward–looking statements reflecting the Company’s current expectations that necessarily involve risks and uncertainties. Actual results and the timing of events may differ materially from those contained in such forward-looking statements due to a number of factors and the Company undertakes no obligation to revise or update any forward-looking statement to reflect events or circumstances after the issuance of this press release.
Contacts
Lucas Nivon
CEO & Co-founder
info@cyrusbio.com




