BOSTON and LEUVEN, Belgium, Nov. 14, 2024 /PRNewswire/ -- Why this news will impact the lives of people affected by Alzheimer's disease:
- The first disease-modifying therapies for Alzheimer's disease have come to the market over the previous years – providing hope to the millions affected by Alzheimer's disease.
- These therapies have been linked to the development of specific brain abnormalities known as Amyloid-Related Imaging Abnormalities (ARIA), which, although rare, can lead to neurological complications.
- Regular brain MRI scans are needed to assess ARIA, but the detection and severity scoring is complex and time-consuming.
- The FDA authorizes icobrain aria as the first and only AI solution to detect, diagnose, and monitor ARIA for individuals with Alzheimer's disease
On November 7th 2024, the U.S. Food and Drug Administration (FDA) granted icometrix clearance for icobrain aria, the first and only AI software approved for detecting, measuring and grading amyloid-related imaging abnormalities (ARIA), a potentially harmful side effect of new amyloid-targeting therapies. A large study, needed for FDA clearance, demonstrated that the use of icobrain aria significantly increases the accuracy of ARIA assessments by radiologists and hence allows for safer use of new amyloid-beta targeting therapies for Alzheimer's disease patients.
"Amyloid-lowering treatments represent an important I am excited that icobrain aria has received FDA | Prof. Stephen Salloway |
The slow adoption of Alzheimer's treatments amid safety concerns
The FDA's nod for icobrain aria comes at a time when much-needed new Alzheimer's treatments are reshaping care options in the United States. The first breakthrough in Alzheimer's disease treatment was in 2021 when aducanumab (Aduhelm) became the first FDA-approved drug intended to slow Alzheimer's progression by reducing amyloid plaques in the brain. This breakthrough, making headlines worldwide, was followed by other therapies, including lecanemab (Leqembi) and donanemab (Kisunla), each designed to target amyloid buildup, which is believed to play a role in Alzheimer's disease. Together, these treatments represent a shift from symptom management to disease-modifying approaches, offering hope to millions of patients and their families.
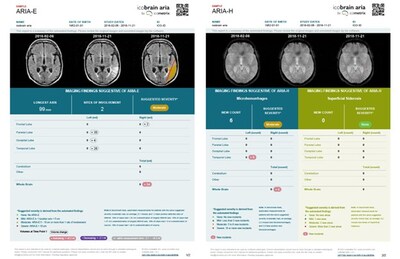
However, these promising drugs also carry risks, one of which is the development of ARIA (amyloid-related imaging abnormalities), a complication that can cause lesions in the brain, including edema, swelling and/or hemorrhages. According to the FDA, the prescription of these amyloid-targeting therapies requires intensive ARIA monitoring with repeated magnetic resonance imaging (MRI) brain scans to detect and assess these changes before they pose serious neurological risks. Until now, identifying and evaluating ARIA has been a labor-intensive process, involving detailed, time-consuming, and visual comparisons of the brain MRI scans by radiologists.
FDA nod for AI software to ensure patient safety
icobrain aria is a unique deep-learning-based AI solution that was trained on thousands of brain MRI scans to accurately detect, diagnose, and monitor ARIA and its severity. The software works in the background and automatically generates an overview report on the ARIA assessment for radiologists using normal clinical MRI scans.
"icobrain aria was thoroughly evaluated in large reader | Prof. Jeffrey Cummings |
"We are thrilled to announce FDA authorization of the first- | Dr. Dirk Smeets |
Impacting care for people with Alzheimer's disease
This FDA clearance is a big step forward in Alzheimer's care, demonstrating how technology can help bring safe and effective treatments to more people. With icobrain aria, physicians now have a powerful tool that helps them make informed treatment decisions, ensuring that patients receive the right care at the right time. This is a clear example of how advanced technology can make a difference in healthcare, supporting doctors and making important new therapies more widely accessible.
"For families facing Alzheimer's disease, the new disease- But to help as many people as possible, we do need to Thanks to the FDA clearance of icobrain aria, Alzheimer's | George Vradenburg |
About icometrix
icometrix is a global leader in AI-driven software for brain imaging, transforming MRI and CT scans into actionable data for neurological conditions such as Alzheimer's, multiple sclerosis, and Parkinson's disease. Used in over 300 hospitals worldwide, icobrain technology is setting new standards in precision brain health, supporting doctors with tools that are FDA-cleared in the U.S. and approved in regions across the globe. With a commitment to advancing safe, effective treatments, icometrix partners with top pharmaceutical and health tech companies to bring innovative solutions to those who need them most.
Media Contact: marketing@icometrix.com or
CEO and CTO
Wim Van Hecke - CEO
wim.vanhecke@icometrix.com
+32 484 92 73 00
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/fda-authorizes-the-first-ai-driven-mri-solution-for-safer-alzheimers-treatment-302305479.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/fda-authorizes-the-first-ai-driven-mri-solution-for-safer-alzheimers-treatment-302305479.html
SOURCE icometrix