First generic version of Epinephrine 1mg/1mL vial in U.S. market
LAKE ZURICH, Ill.--(BUSINESS WIRE)--#FreseniusKabi--Fresenius Kabi, an operating company of Fresenius, announced today that Epinephrine Injection, USP, is now available in the United States as the first generic version of Epinephrine in a 1mg/1mL vial for U.S. customers.
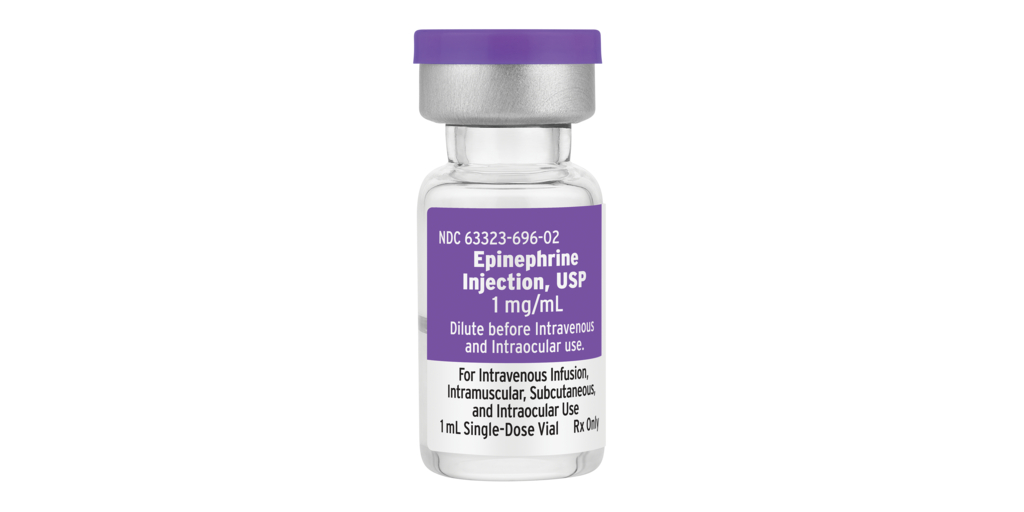


Epinephrine Injection, USP is a prescription medicine used to increase mean arterial blood pressure in adult patients with hypotension associated with septic shock; for emergency treatment of allergic reactions, including anaphylaxis; and for induction and maintenance of mydriasis during intraocular surgery.
“Fresenius Kabi is pleased to offer Epinephrine Injection to our U.S. customers, further demonstrating our continued commitment to providing quality, cost-saving treatment options to clinicians and the patients they serve in line with #FutureFresenius,” said Arunesh Verma, president, Fresenius Kabi Region U.S. and Member, Executive Leadership Team, Fresenius Kabi AG.
Fresenius Kabi has invested nearly $1 billion to expand and update its U.S. pharmaceutical production and distribution facilities. Fresenius Kabi is committed to domestic production. More than 70 percent of the product units Fresenius ships in the U.S. are drugs listed on the FDA’s Essential Medicines List.
INDICATIONS AND USAGE
Epinephrine is a non-selective alpha- and beta-adrenergic agonist indicated:
- To increase mean arterial blood pressure in adult patients with hypotension associated with septic shock.
- For emergency treatment of allergic reactions (Type 1), including anaphylaxis.
- For induction and maintenance of mydriasis during intraocular surgery.
IMPORTANT SAFETY INFORMATION
Select Warnings and Precautions for Epinephrine Injection, USP are as follows:
- Monitor patient for acute severe hypertension.
- Avoid epinephrine extravasation into tissues, which can cause local necrosis.
- Do not inject epinephrine into buttocks, digits, hands, or feet.
- Potential for pulmonary edema when epinephrine is administered intravenously, which may be fatal. Treat with a rapidly acting alpha-adrenergic blocking drug and respiratory support.
- May constrict renal blood vessels and decrease urine formation.
- May induce potentially serious cardiac arrhythmias or aggravate angina pectoris, particularly in patients with underlying heart disease.
- Presence of sulfite in this product should not deter use for treatment of serious allergic or other emergency situations.
Most common adverse reactions to systemically administered epinephrine are headache; anxiety; apprehensiveness; restlessness; tremor; weakness; dizziness; sweating; palpitations; pallor; peripheral coldness; nausea/vomiting; and/or respiratory difficulties. Arrhythmias, including fatal ventricular fibrillation, rapid rises in blood pressure producing cerebral hemorrhage, and angina have occurred.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions:
- Drugs that counter the pressor effects of epinephrine include alpha blockers, vasodilators such as nitrates, diuretics, antihypertensives, and ergot alkaloids.
- Drugs that potentiate the effects of epinephrine include sympathomimetics, beta blockers, tricyclic antidepressants, MAO inhibitors, COMT inhibitors, clonidine, doxapram, oxytocin, levothyroxine sodium, and certain antihistamines.
- Drugs that increase the arrhythmogenic potential of epinephrine include beta blockers, cyclopropane and halogenated hydrocarbon anesthetics, quinidine, antihistamines, exogenous thyroid hormones, diuretics, and cardiac glycosides. Observe for development of cardiac arrhythmias.
- Potassium-depleting drugs, including corticosteroids, diuretics, and theophylline, potentiate the hypokalemic effects of epinephrine.
Pregnancy: May cause fetal harm.
Elderly patients and pregnant women may be at greater risk of developing adverse reactions when epinephrine is administered parenterally.
This Important Safety Information does not include all the information needed to use Epinephrine Injection, USP safely and effectively. Please see full prescribing information for Epinephrine Injection, USP at www.fresenius-kabi.com/us.
About Fresenius Kabi
As a global health care company, Fresenius Kabi is Committed to Life. The company’s products, technologies, and services are used for the therapy and care of patients with critical and chronic conditions. With more than 43,000 employees and present in more than 100 countries, Fresenius Kabi’s expansive product portfolio focuses on providing access to high-quality and lifesaving medicines and technologies.
In Biopharma, Fresenius Kabi offers cutting-edge biosimilars for autoimmune diseases and oncology. With leading market positions in Clinical Nutrition, a broad portfolio of enteral and parenteral products makes a distinct difference in patients’ nutritional status – notably as the only corporation offering both product groups. In MedTech, the company provides vital infusion pumps, cell and gene therapy devices, disposables, and more. Fresenius Kabi is a global leader in supplying blood collection bags and devices, supporting blood banks and health care facilities worldwide. The company’s I.V. Generics and Fluids for infusion therapy help save millions of lives every year, in emergency medicine, surgery, oncology, and intensive care.
Fresenius Kabi takes a holistic approach to health care and uniquely combines experience, expertise, innovation, and dedication – making a difference in the lives of almost 450 million patients annually. With Vision 2026, as part of the #FutureFresenius strategy, the company is developing, producing, and selling new products and technologies and aspires to expand its position as a leading global provider of therapies, improve patient care, generate sustainable value for stakeholders – shaping the future of health care.
Fresenius Kabi is an operating company of the Fresenius Group, founded in 1912, along with Helios and Quirónsalud. As ONE team, the companies in the Fresenius Group are committed to providing lifesaving and life-changing health care solutions on a global scale.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g., changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius Kabi does not undertake any responsibility to update the forward-looking statements in this release.
For more information, please visit http://www.fresenius-kabi.com and http://www.fresenius-kabi.com/us in the United States.
Management Board: Pierluigi Antonelli (Chairman), Marc Crouton, Andreas Duenkel, Dr. Christian Hauer, Dr. Marc-Alexander Mahl, Dr. Sang-Jin Pak
Chairman of the Supervisory Board: Wolfgang Kirsch
Registered Office: Bad Homburg, Germany
Commercial Register: Amtsgericht Bad Homburg - HRB 11654
Contacts
Matt Kuhn (847) 220-3033
matt.kuhn@fresenius-kabi.com




