Hand Eczema Market Outlook 2025-2035:
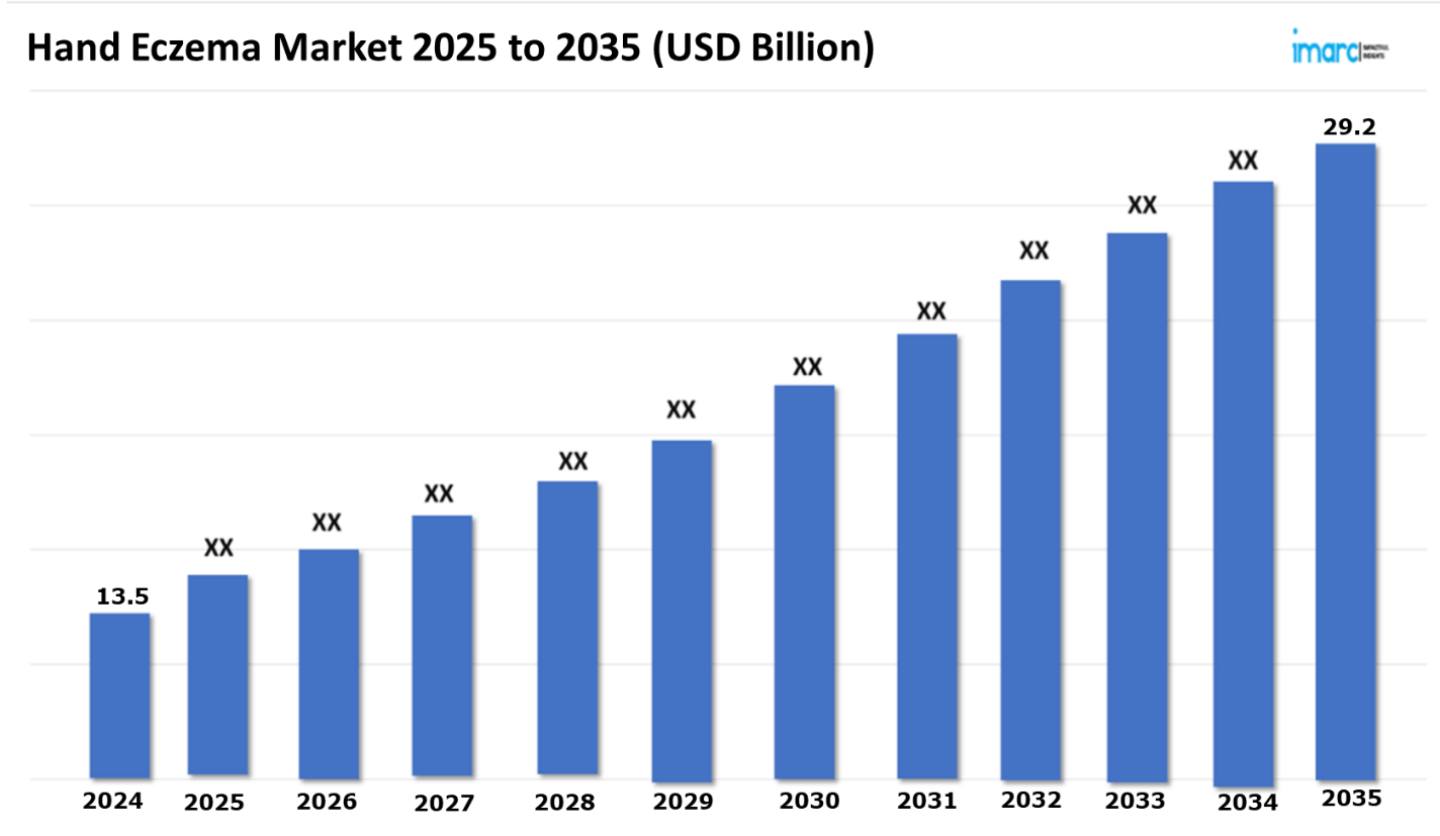
The 7 major hand eczema market reached a value of USD 13.5 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 29.2 Billion by 2035, exhibiting a growth rate (CAGR) of 7.28% during 2025-2035. The Hand Eczema market is growing because of the rising demand for non-invasive and minimally invasive therapies, including topical corticosteroids, emollients, phototherapy, and newer biologics. These treatments effectively address hand eczema symptoms, decrease inflammation, and avert flare-ups while presenting a reduced likelihood of side effects in comparison to conventional therapies. The emergence of biologic medications that focus on particular immune responses related to eczema is transforming therapy for moderate to severe instances, offering prolonged relief and enhancing quality of life. Moreover, improvements in phototherapy, like narrowband UVB therapy, are offering focused treatments that reduce the chance of skin damage while efficiently managing flare-ups. These non-invasive methods are especially appealing to patients who favor treatments that have fewer side effects, quicker recovery periods, and reduced chances of complications. In addition, the rising awareness of hand eczema and access to improved treatment options are leading to greater patient satisfaction and an increasing demand for personalized therapies that address the condition without the need for more invasive approaches. Consequently, these advancements are driving the hand eczema market ahead, concentrating on enhancing patient results while reducing the necessity for prolonged medication usage and invasive treatments.
Advances in Early Detection and Diagnostic Technologies: Driving the Hand Eczema Market
Contemporary diagnostic and therapeutic technologies are greatly altering the Hand Eczema market, with the goal of enhancing patient care and results. Sophisticated imaging methods like dermoscopy and high-resolution photography allow for precise visualization and ongoing observation of hand eczema lesions, aiding in accurate evaluation and treatment strategies. Backing these innovations are diagnostic instruments like sebum analysis and skin microbiome profiling, which aid in identifying the underlying factors of hand eczema, such as bacterial overgrowth or inflammatory responses. Molecular diagnostics, such as PCR and next-generation sequencing (NGS), are becoming significant tools for identifying important pathogens and examining the skin’s microbiome, facilitating more tailored treatment strategies. The incorporation of artificial intelligence (AI) into imaging and diagnostic systems enhances accuracy by facilitating automatic categorization and severity evaluations of eczema lesions. This technology diminishes dependence on personal assessments, boosting the precision of monitoring lesion therapy advancement and ultimately enhancing treatment results. Non-invasive treatments like advanced laser therapies, photodynamic therapy, and chemical peels are becoming increasingly popular because they require less recovery time and have fewer negative side effects. These treatments provide effective control of eczema outbreaks while promoting quicker healing and enhanced patient contentment. Additionally, the rise of novel wearable technologies, such as smart patches, provides real-time monitoring of skin conditions, offering tailored treatment solutions. These innovations are particularly valuable in regions where access to dermatologists is limited, allowing for prompt recognition of skin changes and more individualized treatment plans. This method aids in reducing the chances of scarring and emotional distress, fostering improved long-term handling of the condition. Telemedicine platforms are essential for increasing access to dermatological care by enabling remote consultations, diagnoses, and treatment suggestions. These platforms guarantee that patients obtain prompt care no matter their geographic location, which further aids the market’s expansion and enhances access to advanced treatment alternatives. Together, these modern technologies are reshaping the hand eczema landscape, offering patients effective, personalized care and fostering better long-term outcomes.
Request a PDF Sample Report: https://www.imarcgroup.com/hand-eczema-market/requestsample
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The Hand Eczema market is witnessing notable expansion due to the launch of innovative therapies alongside improved pharmacological treatments. Novel topical and oral treatments are in development to address resistant strains of pathogens and the root causes of hand eczema, rendering them vital for both the prevention and treatment of the condition. These new medications show enhanced effectiveness, fewer side effects, and a more precise mechanism of action, resulting in greater patient satisfaction and improved treatment results. Investigation into biological medications is growing swiftly, especially for addressing moderate to severe instances of hand eczema, particularly in chronic inflammatory disorders. These biologics consist of monoclonal antibodies that focus on pro-inflammatory cytokines like interleukin-17 and interleukin-1. These therapies offer a hopeful approach for better managing hand eczema by targeting both bacterial activity and the inflammation processes linked to the condition. Innovations in drug delivery systems, such as liposomal formulations, hydrogels, and carriers based on nanotechnology, are transforming the treatment paradigm. These systems facilitate targeted delivery of therapeutic agents, guaranteeing a greater concentration of the medication at the action site, while decreasing systemic exposure and lowering potential side effects. Treatments being developed as adjuncts focus on reestablishing the skin’s microbial balance and enhancing the body’s innate defenses against hand eczema. Probiotics and immunomodulators are receiving increased interest as adjunctive treatments that may improve the skin’s durability and avert flare-ups. Combination therapies that merge antimicrobials with anti-inflammatory medications or retinoids show potential in tackling the complex pathophysiology of hand eczema by offering a holistic treatment strategy. Moreover, non-invasive medicinal options, like biofilm-disrupting substances and innovative topical preparations, are becoming increasingly favored for their straightforward application and focus on patient needs. These therapies provide patients with a more accessible and efficient method to control their condition, aiding in the market’s expansion and enhancing overall treatment compliance.
Buy Full Report: https://www.imarcgroup.com/checkout?id=11209&method=809
Marketed Therapies in Hand Eczema Market
Toctino (Alitretinoin oral): Stiefel Laboratories
Toctino (alitretinoin) is an oral retinoid that is approved for treating severe hand eczema (chronic hand dermatitis) in adults unresponsive to topical therapies. It functions by adjusting the immune system and enhancing skin cell renewal, aiding in the reduction of inflammation and bettering skin lesions related to the condition.
Emerging Therapies in the Hand Eczema Market
LEO 124249: Japan Tobacco/LEO Pharma
LEO 124249 is a research topical therapy for hand eczema that focuses on the root inflammatory mechanisms of the ailment. Presently in clinical trials, it seeks to serve as an effective substitute for conventional corticosteroids, potentially delivering a safer and more focused treatment choice for individuals with hand eczema.
Dupilumab: Regeneron/Sanofi
Dupilumab is a monoclonal antibody utilized for managing moderate-to-severe hand eczema, particularly when topical therapies are inadequate. It functions by blocking essential inflammatory pathways, particularly focusing on interleukin-4 and interleukin-13, which are crucial in the inflammation and symptoms associated with eczema. This biological treatment has demonstrated efficacy in diminishing flare-ups and enhancing skin conditions in individuals with hand eczema.
AZT 03: Azitra
AZT 03 is an investigational drug being studied for its potential to treat inflammatory skin conditions, including hand eczema. It works by targeting specific immune pathways involved in the inflammation process, offering a promising treatment option for patients with moderate to severe hand eczema. However, it is still undergoing clinical trials to assess its safety and efficacy.
Drug Name | Company Name | MOA | ROA |
LEO 124249 | LEO Pharma | Janus kinase inhibitors | Topical |
Dupilumab | Regeneron/Sanofi | Interleukin 13 receptor antagonists; Interleukin 4 receptor antagonists | Subcutaneous |
AZT 03 | Azitra | Interleukin 10 modulators; Microbiome modulators; S100 protein modulators | Topical |
Detailed list of emerging therapies in Hand Eczema is provided in the final report…
Leading Companies in the Hand Eczema Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global Hand Eczema market, several leading companies are at the forefront of developing integrated platforms to enhance the management of Hand Eczema. Some of the major players include LEO Pharma, Regeneron/Sanofi, Azitra, Stiefel Laboratories, and others. These companies are driving innovation in the Hand Eczema market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for Hand Eczema.
In September 2024, LEO Pharma Inc. announced that the U.S. Food and Drug Administration (FDA) has accepted the submission of a New Drug Application (NDA) for delgocitinib cream 20 mg/g (2%) aimed at treating adults with moderate to severe Chronic Hand Eczema (CHE), especially for those who have not sufficiently responded to other therapies or for whom topical corticosteroids are not advisable.
Key Players in Hand Eczema Market:
The key players in the Hand Eczema market who are in different phases of developing different therapies are LEO Pharma, Regeneron/Sanofi, Azitra, Stiefel Laboratories, and Others.
Regional Analysis:
The major markets for Hand Eczema include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for Hand Eczema while also representing the biggest market for its treatment. Recent progress in hand eczema treatments features the introduction of innovative therapies like advanced retinoids, antimicrobial peptides, biofilm-disrupting agents, and hormonal modulators, providing more precise and effective alternatives. These therapies focus on tackling the root causes of hand eczema, including inflammation, excessive bacteria, and skin barrier impairment, leading to improved results with reduced negative effects.
Simultaneously, advancements in diagnostic tools and methods have enhanced the precision and rapidity of hand eczema diagnosis, facilitating earlier intervention and tailored treatment approaches. These developments enable more accurate targeting of the condition’s underlying causes, reducing the likelihood of complications. Elements driving the expansion of the hand eczema market encompass greater funding in research and development, the endorsement of new treatment guidelines, and improved cooperation among pharmaceutical firms, diagnostic technology suppliers, and research organizations. Diagnostic tools driven by AI and telemedicine platforms are crucial in enhancing access to advanced treatments, especially in rural or neglected areas. As these technologies and treatments progress, areas such as North America and Europe continue to be at the forefront of hand eczema innovation, propelling worldwide market expansion and enhancing patient results globally.
Recent Developments in Hand Eczema Market:
· In July 2024, LEO Pharmaceuticals announced positive results from two Phase 3 trials evaluating the safety and efficacy of delgocitinib cream used twice daily for adult patients with moderate to severe chronic hand eczema (CHE). The DELTA 1 and DELTA 2 studies demonstrated that delgocitinib cream successfully achieved its primary objective, achieving an Investigator’s Global Assessment for effective chronic hand eczema treatment (IGA-CHE TS) score of 0 or 1. Moreover, the therapy achieved all major secondary endpoints, demonstrating a notable decrease in itching and discomfort, as indicated by scores of 4 or less on the Hand Eczema Symptom Diary (HESD).
Key information covered in the report.
Base Year: 2024
Historical Period: 2019-2024
Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the Hand Eczema market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the Hand Eczema market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current Hand Eczema marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/hand-eczema-market/toc
IMARC Group Offer Other Reports:
Panuveitis Market: The 7 major panuveitis markets are expected to exhibit a CAGR of 7.49% during 2025-2035.
Atopic Dermatitis Market: The 7 major atopic dermatitis markets reached a value of US$ 16,816.8 Million in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 37,213.7 Million by 2034, exhibiting a growth rate (CAGR) of 7.49% during 2024-2034.
Invasive Pneumococcal Disease Market: The 7 major invasive pneumococcal disease markets reached a value of US$ 6.3 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 11.0 Billion by 2034, exhibiting a growth rate (CAGR) of 5.13% during 2024-2034.
Contact Dermatitis Market: The 7 major contact dermatitis markets reached a value of US$ 10.8 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 19.4 Billion by 2034, exhibiting a growth rate (CAGR) of 5.46% during 2024-2034.
Hidradenitis Suppurativa Market: The 7 major hidradenitis suppurativa markets reached a value of US$ 1.4 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 3.9 Billion by 2034, exhibiting a growth rate (CAGR) of 9.79% during 2024-2034.
Palmar Hyperhidrosis Market: The 7 major palmar hyperhidrosis markets reached a value of US$ 231.1 Million in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 352.6 Million by 2034, exhibiting a growth rate (CAGR) of 3.92% during 2024-2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800