Hidradenitis Suppurativa Market Outlook 2025-2035:
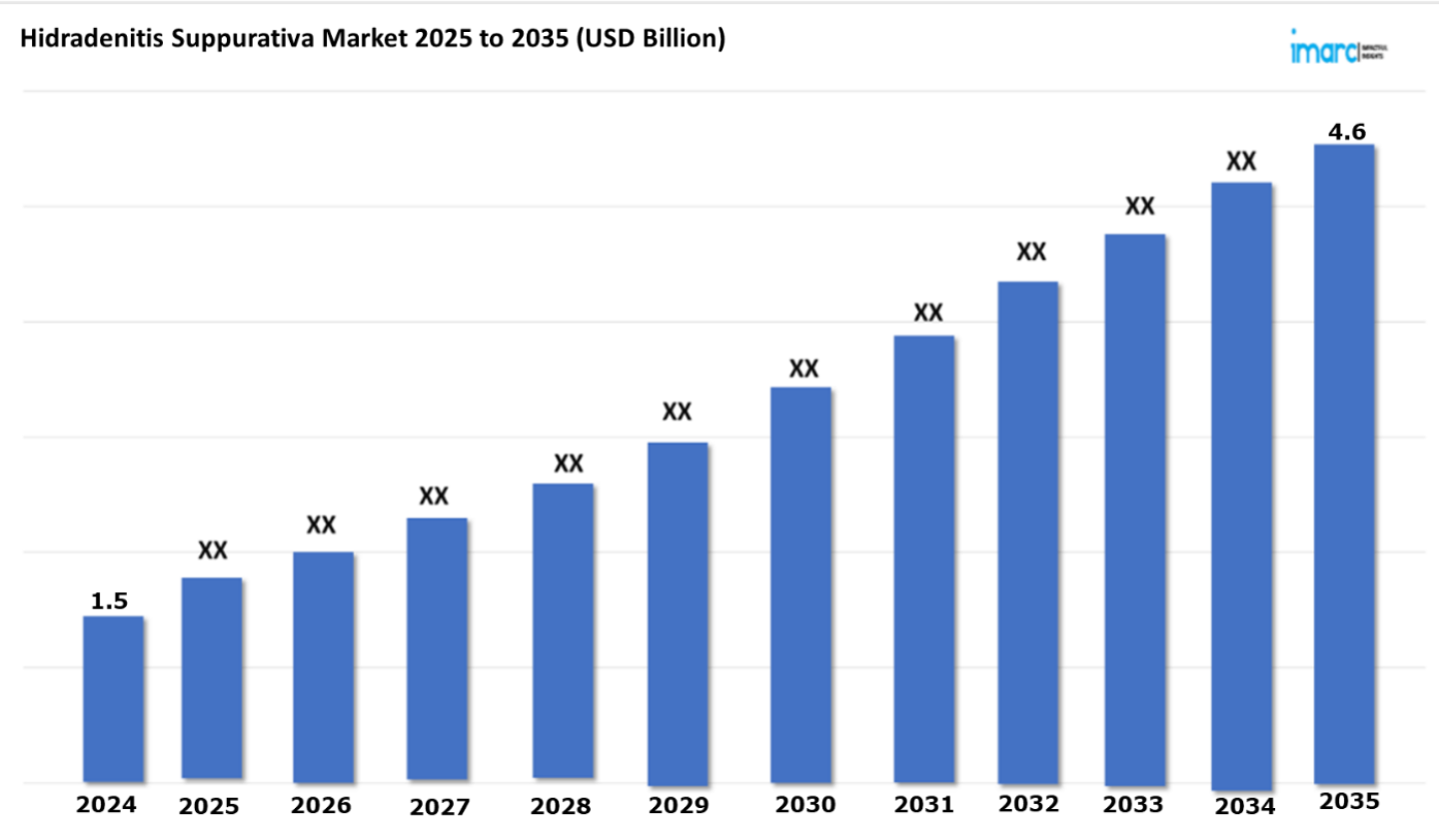
The 7 major hidradenitis suppurativa market reached a value of USD 1.5 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 4.6 Billion by 2035, exhibiting a growth rate (CAGR) of 10.55% during 2025-2035. The market for hidradenitis suppurativa (HS) treatment shows significant growth because of improved diagnoses, better treatment options, and heightened patient and medical professional awareness of the condition. The demand for innovative therapies increases because of the growing incidence of the condition that specifically affects skin areas like the armpits and groin. The market also expands because healthcare providers implement novel biologic therapies that treat inflammation and deliver beneficial results to patients' quality of life. Research that investigates HS pathophysiology enables better development of effective treatments. Moreover, the market expansion is supported by rising healthcare accessibility, increasing focus on dermatology, and improved accessibility to healthcare.
Awareness Expansion and Better Treatment Access: Driving the Hidradenitis Suppurativa Market
The hidradenitis suppurativa (HS) market is experiencing notable growth due to increased awareness and better access to treatment options. The increased comprehension of the condition leads patients and healthcare professionals to learn more about HS symptoms and their causes and treatment options. Public awareness efforts delivered by both medical organizations and patient support groups and their educational outreach strategies have diminished misunderstandings about HS thus leading more people to obtain medical help. This increased public awareness has led to more identified HS cases thus boosting the need for appropriate treatment. The expansion of specialized dermatology care services became possible through healthcare service improvements in developing regions. Healthcare institutions along with increased insurance access allow more patients to get essential HS treatments from traditional antibiotic care to advanced biologic medications which attack inflammatory responses. The pharmaceutical industry has directed its efforts toward developing novel HS treatments because of growing medical interest in this condition. Biologic treatments that deal with HS root causes have brought a new standard to disease management while providing better relief options to patients. The development of new diagnostic approaches enables doctors to find HS early thus initiating rapid treatment to boost patient recovery rates. The HS market continues to grow because patient awareness of HS is rising while medical specialists provide better treatment options. The market will advance because more patients receive early diagnoses together with advanced medical interventions which will boost future treatment requirements.
Request a PDF Sample Report: https://www.imarcgroup.com/hidradenitis-suppurativa-market/requestsample
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The development of new pharmaceutical therapies and drugs dedicated to hidradenitis suppurativa (HS) treatment has substantially expanded the HS market. The recent development of new treatment possibilities results in improved HS patient outcomes. Users of TNF inhibitors have shown successful results in controlling disease symptoms along with reducing their flare-up frequency. The use of interimila LINs (IL-12/23 inhibitors) and JAK inhibitors maintains a promising perspective by providing unique therapeutic options to treat HS. These drugs provide better treatment outcomes, which minimizes the adverse effects to positively impact patient quality of life and resolve a medical demand that existed over time. The pharmaceutical field is developing both oral and topical medications which enable doctors to create personalized treatment regimens. Advanced therapies experience rising demand because healthcare professionals together with patients recognize HS increasingly. A large number of pharmaceutical companies dedicate their resources toward research and development activities which lead to competition enhancement and market modernization. The regulatory process behind adalimumab alongside other biologics allowed medical providers to receive new treatment options more quickly. Medical professionals from dermatology, immunology, and wound care specialty areas are now collaborating to deliver full-spectrum care for patients because of this development. The market growth will receive additional momentum through this joint working model. The market will gain increased investment in HS treatments due to new patient-centered approaches combined with improved outcomes. The market has experienced new possibilities and improved treatment accessibility through innovative therapies that benefit patients by increasing their level of satisfaction.
Buy Full Report: https://www.imarcgroup.com/checkout?id=7127&method=809
Marketed Therapies in Hidradenitis Suppurativa Market
Bimzelx (Bimekizumab): UCB
The monoclonal antibody Bimzelx (bimekizumab) serves as a therapeutic medication for adults who have moderate to severe hidradenitis suppurativa (HS) and was developed by UCB. The medication inhibits both IL-17A and IL-17F interleukins which are essential inflammatory compounds in HS disease processes. Bimzelx decreases HS symptom severity which leads to enhanced skin health along with improved patient life quality for those living with this persistent skin disorder.
Humira (Adalimumab): AbbVie/AstraZeneca
The biologic medication Humira (adalimumab) originated from AbbVie/AstraZeneca as an approved treatment option for adults with moderate to severe hidradenitis suppurativa (HS). The medication works by blocking tumor necrosis factor (TNF) which plays a role in the inflammatory process that causes HS. Through its mechanism Humira controls HS flare-ups and enhances patients' skin health together with their quality of life in treating this persistent skin disease.
Cosentyx (Secukinumab): Novartis
The pharmaceutical company Novartis developed Cosentyx (secukinumab) for treating adults with moderate to severe hidradenitis suppurativa (HS) through its role as a biologic medication. The drug prevents interleukin-17A (IL-17A) activity along with blocking inflammatory processes in HS. The FDA authorized Cosentyx as the initial whole human biologic treatment for HS to deliver patients a novel therapeutic approach for their persistent skin condition.
Emerging Therapies in Hidradenitis Suppurativa Market
Lutikizumab: AbbVie
The pharmaceutical company AbbVie developed Lutikizumab (ABT-981) as a new investigational biologic treatment for patients with moderate to severe hidradenitis suppurativa (HS). The treatment blocks interleukins 1α and 1β allowing them to prevent inflammation in HS patients. Clinical research demonstrates that lutikizumab provides effective solutions for HS patients whose condition does not respond to anti-TNF medications. Scientists are conducting Phase 3 clinical trials with the therapy as part of its current advancement.
Povorcitinib: Incyte Corporation
Povorcitinib (INCB054707) is an investigational oral Janus kinase 1 (JAK1) inhibitor developed by Incyte Corporation for the treatment of moderate to severe hidradenitis suppurativa (HS). The investigational drug povorcitinib attacks JAK1 which plays a central role in inflammatory reactions to achieve symptom reduction in HS patients. The drug has displayed promising clinical effectiveness in early trials as it progresses through Phase 3 studies to determine long-term safety and effectiveness for HS patients.
Opzelura (Ruxolitinib cream): Incyte Corporation
Ruxolitinib cream (Opzelura), developed by Incyte Corporation, is a topical Janus kinase (JAK) inhibitor designed for the treatment of mild-to-moderate hidradenitis suppurativa (HS). Patients should use the topical cream twice per day to block enzymes which govern HS inflammatory responses. Research trials demonstrate that applying ruxolitinib cream successfully decreases both abscesses and inflammatory nodules providing HS patients with a fresh topical treatment method.
Detailed list of emerging therapies in Hidradenitis Suppurativa is provided in the final report…
Leading Companies in the Hidradenitis Suppurativa Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global hidradenitis suppurativa market, several leading companies are at the forefront of developing integrated platforms to enhance the management of hidradenitis suppurativa. Some of the major players include UCB, AbbVie, Incyte Corporation, Novartis, and others. These companies are driving innovation in the hidradenitis suppurativa market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for hidradenitis suppurativa.
In September 2024, The Phase 3 BE HEARD I and BE HEARD II clinical studies of UCB demonstrated through their extended open-label phase that bimekizumab proved effective for treating moderate-to-severe hidradenitis suppurativa (HS) in adults. The data from research was presented at the 33rd European Academy of Dermatology and Venereology (EADV) Congress Amsterdam, Netherlands through a late-breaking platform presentation during September 25–28, 2024.
Key Players in Hidradenitis Suppurativa Market:
The key players in the hidradenitis suppurativa market who are in different phases of developing different therapies are Novartis, UCB, Incyte Corporation, AbbVie, Janssen Biotech, AstraZeneca, and others.
Regional Analysis:
The major markets for hidradenitis suppurativa include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for hidradenitis suppurativa while also representing the biggest market for its treatment. Recent progress in treating hidradenitis suppurativa (HS) has focused on more targeted therapies that address the inflammation driving the condition. The approval of biologics like bimekizumab, which blocks both IL-17A and IL-17F, represents a major advancement in managing moderate-to-severe HS. Other biologics, including adalimumab have also shown promising results. Research is now delving into the role of the microbiome in HS, which could lead to new treatment options. With better early detection and personalized treatment strategies, the quality of life for patients with this chronic, often painful condition is improving.
Recent Developments in Hidradenitis Suppurativa Market:
· In January 2025, UCB announced the availability of a new 2 mL prefilled syringe and autoinjector for Bimzelx (bimekizumab-bkzx), each containing 320 mg of the medication. These new options complement the existing 1 mL administration, which contains 160 mg of Bimzelx.
· In November 2024, UCB acquired regulatory approval from the FDA to market Bimzelx (bimekizumab-bkzx) for HS treatment in adults with moderate-to-severe hidradenitis suppurativa (HS). Bimzelx represents the initial drug solution that blocks both interleukin 17A (IL-17A) and interleukin 17F (IL-17F).
· In March 2024, Incyte presented Phase 2 study findings at the Hurley stage 1 or 2 HS patients evaluating ruxolitinib cream 1.5% effectiveness (Opzelura®) when used twice daily. Ruxolitinib cream 1.5% (Opzelura) data was shown as a late-breaking oral session (S050 - Late-Breaking Research: Session 2) at the American Academy of Dermatology (AAD) Annual Meeting held March 8-12, 2024 in San Diego.
· In January 2024, Research from AbbVie's Phase 2 trial demonstrated better clinical outcomes with lutikizumab (ABT-981) in adults having moderate to severe hidradenitis suppurativa (HS) although they had not responded to anti-TNF therapies in the past. The main study goal for significant HS symptom reduction at week 16 was reached by patients taking 300 mg doses of lutikizumab either weekly or every other week while showing better results than placebo recipients. The phase 3 clinical trials for lutikizumab HS therapy will progress because AbbVie approved the outcomes from the trials.
· In October 2023, Novartis declared their submission of Cosentyx (secukinumab) to the U.S. Food and Drug Administration (FDA) for treating moderate to severe hidradenitis suppurativa (HS) in adults. The FDA granted approval for Cosentyx as the initial fully human biologic to inhibit interleukin-17A (IL-17A) cytokines which makes essential contributions to HS inflammatory processes.
Key information covered in the report.
Base Year: 2024
Historical Period: 2019-2024
Market Forecast: 2025-2035
Countries Covered
This report offers a comprehensive analysis of current hidradenitis suppurativa marketed drugs and late-stage pipeline drugs.
In-Market Drugs
IMARC Group Offer Other Reports:
Bile Duct Diseases Market: The 7 major bile duct diseases markets are expected to exhibit a CAGR of 8.21% during 2024-2034.
Cardiac Arrhythmias Market: The 7 major cardiac arrhythmias markets reached a value of US$ 4.9 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 8.0 Billion by 2034, exhibiting a growth rate (CAGR) of 4.57% during 2024-2034.
Acne Vulgaris Market: The 7 major acne vulgaris markets reached a value of USD 7.2 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 10.4 Billion by 2035, exhibiting a growth rate (CAGR) of 3.33% during 2025-2035.
Psoriasis Market: The psoriasis market reached a value of US$ 19.2 Billion across the top 7 markets (US, EU4, UK, and Japan) in 2023. Looking forward, IMARC Group expects the top 7 markets to reach US$ 35.8 Billion by 2034, exhibiting a growth rate (CAGR) of 5.85% during 2024-2034.
Atopic Dermatitis Market: The 7 major atopic dermatitis markets reached a value of US$ 16,816.8 Million in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 37,213.7 Million by 2034, exhibiting a growth rate (CAGR) of 7.49% during 2024-2034.
Eczema Market: The 7 major eczema markets reached a value of US$ 16.8 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 37.1 Billion by 2034, exhibiting a growth rate (CAGR) of 7.46% during 2024-2034.
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
The 7 major hidradenitis suppurativa market reached a value of USD 1.5 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 4.6 Billion by 2035, exhibiting a growth rate (CAGR) of 10.55% during 2025-2035. The market for hidradenitis suppurativa (HS) treatment shows significant growth because of improved diagnoses, better treatment options, and heightened patient and medical professional awareness of the condition. The demand for innovative therapies increases because of the growing incidence of the condition that specifically affects skin areas like the armpits and groin. The market also expands because healthcare providers implement novel biologic therapies that treat inflammation and deliver beneficial results to patients' quality of life. Research that investigates HS pathophysiology enables better development of effective treatments. Moreover, the market expansion is supported by rising healthcare accessibility, increasing focus on dermatology, and improved accessibility to healthcare.
Awareness Expansion and Better Treatment Access: Driving the Hidradenitis Suppurativa Market
The hidradenitis suppurativa (HS) market is experiencing notable growth due to increased awareness and better access to treatment options. The increased comprehension of the condition leads patients and healthcare professionals to learn more about HS symptoms and their causes and treatment options. Public awareness efforts delivered by both medical organizations and patient support groups and their educational outreach strategies have diminished misunderstandings about HS thus leading more people to obtain medical help. This increased public awareness has led to more identified HS cases thus boosting the need for appropriate treatment. The expansion of specialized dermatology care services became possible through healthcare service improvements in developing regions. Healthcare institutions along with increased insurance access allow more patients to get essential HS treatments from traditional antibiotic care to advanced biologic medications which attack inflammatory responses. The pharmaceutical industry has directed its efforts toward developing novel HS treatments because of growing medical interest in this condition. Biologic treatments that deal with HS root causes have brought a new standard to disease management while providing better relief options to patients. The development of new diagnostic approaches enables doctors to find HS early thus initiating rapid treatment to boost patient recovery rates. The HS market continues to grow because patient awareness of HS is rising while medical specialists provide better treatment options. The market will advance because more patients receive early diagnoses together with advanced medical interventions which will boost future treatment requirements.
Request a PDF Sample Report: https://www.imarcgroup.com/hidradenitis-suppurativa-market/requestsample
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The development of new pharmaceutical therapies and drugs dedicated to hidradenitis suppurativa (HS) treatment has substantially expanded the HS market. The recent development of new treatment possibilities results in improved HS patient outcomes. Users of TNF inhibitors have shown successful results in controlling disease symptoms along with reducing their flare-up frequency. The use of interimila LINs (IL-12/23 inhibitors) and JAK inhibitors maintains a promising perspective by providing unique therapeutic options to treat HS. These drugs provide better treatment outcomes, which minimizes the adverse effects to positively impact patient quality of life and resolve a medical demand that existed over time. The pharmaceutical field is developing both oral and topical medications which enable doctors to create personalized treatment regimens. Advanced therapies experience rising demand because healthcare professionals together with patients recognize HS increasingly. A large number of pharmaceutical companies dedicate their resources toward research and development activities which lead to competition enhancement and market modernization. The regulatory process behind adalimumab alongside other biologics allowed medical providers to receive new treatment options more quickly. Medical professionals from dermatology, immunology, and wound care specialty areas are now collaborating to deliver full-spectrum care for patients because of this development. The market growth will receive additional momentum through this joint working model. The market will gain increased investment in HS treatments due to new patient-centered approaches combined with improved outcomes. The market has experienced new possibilities and improved treatment accessibility through innovative therapies that benefit patients by increasing their level of satisfaction.
Buy Full Report: https://www.imarcgroup.com/checkout?id=7127&method=809
Marketed Therapies in Hidradenitis Suppurativa Market
Bimzelx (Bimekizumab): UCB
The monoclonal antibody Bimzelx (bimekizumab) serves as a therapeutic medication for adults who have moderate to severe hidradenitis suppurativa (HS) and was developed by UCB. The medication inhibits both IL-17A and IL-17F interleukins which are essential inflammatory compounds in HS disease processes. Bimzelx decreases HS symptom severity which leads to enhanced skin health along with improved patient life quality for those living with this persistent skin disorder.
Humira (Adalimumab): AbbVie/AstraZeneca
The biologic medication Humira (adalimumab) originated from AbbVie/AstraZeneca as an approved treatment option for adults with moderate to severe hidradenitis suppurativa (HS). The medication works by blocking tumor necrosis factor (TNF) which plays a role in the inflammatory process that causes HS. Through its mechanism Humira controls HS flare-ups and enhances patients' skin health together with their quality of life in treating this persistent skin disease.
Cosentyx (Secukinumab): Novartis
The pharmaceutical company Novartis developed Cosentyx (secukinumab) for treating adults with moderate to severe hidradenitis suppurativa (HS) through its role as a biologic medication. The drug prevents interleukin-17A (IL-17A) activity along with blocking inflammatory processes in HS. The FDA authorized Cosentyx as the initial whole human biologic treatment for HS to deliver patients a novel therapeutic approach for their persistent skin condition.
Emerging Therapies in Hidradenitis Suppurativa Market
Lutikizumab: AbbVie
The pharmaceutical company AbbVie developed Lutikizumab (ABT-981) as a new investigational biologic treatment for patients with moderate to severe hidradenitis suppurativa (HS). The treatment blocks interleukins 1α and 1β allowing them to prevent inflammation in HS patients. Clinical research demonstrates that lutikizumab provides effective solutions for HS patients whose condition does not respond to anti-TNF medications. Scientists are conducting Phase 3 clinical trials with the therapy as part of its current advancement.
Povorcitinib: Incyte Corporation
Povorcitinib (INCB054707) is an investigational oral Janus kinase 1 (JAK1) inhibitor developed by Incyte Corporation for the treatment of moderate to severe hidradenitis suppurativa (HS). The investigational drug povorcitinib attacks JAK1 which plays a central role in inflammatory reactions to achieve symptom reduction in HS patients. The drug has displayed promising clinical effectiveness in early trials as it progresses through Phase 3 studies to determine long-term safety and effectiveness for HS patients.
Opzelura (Ruxolitinib cream): Incyte Corporation
Ruxolitinib cream (Opzelura), developed by Incyte Corporation, is a topical Janus kinase (JAK) inhibitor designed for the treatment of mild-to-moderate hidradenitis suppurativa (HS). Patients should use the topical cream twice per day to block enzymes which govern HS inflammatory responses. Research trials demonstrate that applying ruxolitinib cream successfully decreases both abscesses and inflammatory nodules providing HS patients with a fresh topical treatment method.
| Drug Name | Company Name | MOA | ROA |
| Lutikizumab | AbbVie | Interleukin 1 alpha inhibitors; Interleukin 1 beta inhibitors | Subcutaneous |
| Povorcitinib | Incyte Corporation | Janus kinase 1 inhibitors | Oral |
| Opzelura (Ruxolitinib cream) | Incyte Corporation | Janus kinase 1 inhibitors; Janus kinase 2 inhibitors | Topical Cream |
Leading Companies in the Hidradenitis Suppurativa Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global hidradenitis suppurativa market, several leading companies are at the forefront of developing integrated platforms to enhance the management of hidradenitis suppurativa. Some of the major players include UCB, AbbVie, Incyte Corporation, Novartis, and others. These companies are driving innovation in the hidradenitis suppurativa market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for hidradenitis suppurativa.
In September 2024, The Phase 3 BE HEARD I and BE HEARD II clinical studies of UCB demonstrated through their extended open-label phase that bimekizumab proved effective for treating moderate-to-severe hidradenitis suppurativa (HS) in adults. The data from research was presented at the 33rd European Academy of Dermatology and Venereology (EADV) Congress Amsterdam, Netherlands through a late-breaking platform presentation during September 25–28, 2024.
Key Players in Hidradenitis Suppurativa Market:
The key players in the hidradenitis suppurativa market who are in different phases of developing different therapies are Novartis, UCB, Incyte Corporation, AbbVie, Janssen Biotech, AstraZeneca, and others.
Regional Analysis:
The major markets for hidradenitis suppurativa include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for hidradenitis suppurativa while also representing the biggest market for its treatment. Recent progress in treating hidradenitis suppurativa (HS) has focused on more targeted therapies that address the inflammation driving the condition. The approval of biologics like bimekizumab, which blocks both IL-17A and IL-17F, represents a major advancement in managing moderate-to-severe HS. Other biologics, including adalimumab have also shown promising results. Research is now delving into the role of the microbiome in HS, which could lead to new treatment options. With better early detection and personalized treatment strategies, the quality of life for patients with this chronic, often painful condition is improving.
Recent Developments in Hidradenitis Suppurativa Market:
· In January 2025, UCB announced the availability of a new 2 mL prefilled syringe and autoinjector for Bimzelx (bimekizumab-bkzx), each containing 320 mg of the medication. These new options complement the existing 1 mL administration, which contains 160 mg of Bimzelx.
· In November 2024, UCB acquired regulatory approval from the FDA to market Bimzelx (bimekizumab-bkzx) for HS treatment in adults with moderate-to-severe hidradenitis suppurativa (HS). Bimzelx represents the initial drug solution that blocks both interleukin 17A (IL-17A) and interleukin 17F (IL-17F).
· In March 2024, Incyte presented Phase 2 study findings at the Hurley stage 1 or 2 HS patients evaluating ruxolitinib cream 1.5% effectiveness (Opzelura®) when used twice daily. Ruxolitinib cream 1.5% (Opzelura) data was shown as a late-breaking oral session (S050 - Late-Breaking Research: Session 2) at the American Academy of Dermatology (AAD) Annual Meeting held March 8-12, 2024 in San Diego.
· In January 2024, Research from AbbVie's Phase 2 trial demonstrated better clinical outcomes with lutikizumab (ABT-981) in adults having moderate to severe hidradenitis suppurativa (HS) although they had not responded to anti-TNF therapies in the past. The main study goal for significant HS symptom reduction at week 16 was reached by patients taking 300 mg doses of lutikizumab either weekly or every other week while showing better results than placebo recipients. The phase 3 clinical trials for lutikizumab HS therapy will progress because AbbVie approved the outcomes from the trials.
· In October 2023, Novartis declared their submission of Cosentyx (secukinumab) to the U.S. Food and Drug Administration (FDA) for treating moderate to severe hidradenitis suppurativa (HS) in adults. The FDA granted approval for Cosentyx as the initial fully human biologic to inhibit interleukin-17A (IL-17A) cytokines which makes essential contributions to HS inflammatory processes.
Key information covered in the report.
Base Year: 2024
Historical Period: 2019-2024
Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the hidradenitis suppurativa market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the hidradenitis suppurativa market
- Reimbursement scenario in the market
- In-market and pipeline drugs
This report offers a comprehensive analysis of current hidradenitis suppurativa marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
IMARC Group Offer Other Reports:
Bile Duct Diseases Market: The 7 major bile duct diseases markets are expected to exhibit a CAGR of 8.21% during 2024-2034.
Cardiac Arrhythmias Market: The 7 major cardiac arrhythmias markets reached a value of US$ 4.9 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 8.0 Billion by 2034, exhibiting a growth rate (CAGR) of 4.57% during 2024-2034.
Acne Vulgaris Market: The 7 major acne vulgaris markets reached a value of USD 7.2 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 10.4 Billion by 2035, exhibiting a growth rate (CAGR) of 3.33% during 2025-2035.
Psoriasis Market: The psoriasis market reached a value of US$ 19.2 Billion across the top 7 markets (US, EU4, UK, and Japan) in 2023. Looking forward, IMARC Group expects the top 7 markets to reach US$ 35.8 Billion by 2034, exhibiting a growth rate (CAGR) of 5.85% during 2024-2034.
Atopic Dermatitis Market: The 7 major atopic dermatitis markets reached a value of US$ 16,816.8 Million in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 37,213.7 Million by 2034, exhibiting a growth rate (CAGR) of 7.49% during 2024-2034.
Eczema Market: The 7 major eczema markets reached a value of US$ 16.8 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 37.1 Billion by 2034, exhibiting a growth rate (CAGR) of 7.46% during 2024-2034.
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800