Immix Biopharma Announces Positive U.S. Clinical Data From First Four Patients in NEXICART-2 U.S. Trial of sterically-optimized CAR-T NXC-201 in relapsed/refractory Light Chain (AL) Amyloidosis
- All four patients treated with NXC-201 normalized their disease markers within 30 days of dosing, of which, two are already classified as complete responders (CR), and the remaining two are bone marrow MRD negative (10-6); all patients remain in response as of the data cutoff of Nov 14, 2024
- Bone marrow MRD negativity predicts future CR; company believes remaining two patients could be confirmed as CRs in the coming weeks and months
- Company to provide next program update in H1 2025
- Conference call to discuss results Dec 19 at 11:00 a.m. ET (link to attend)
LOS ANGELES, CA, Dec. 19, 2024 (GLOBE NEWSWIRE) -- Immix Biopharma, Inc. (“ImmixBio”, “Company”, “We” or “Us” or ”IMMX”), a clinical-stage biopharmaceutical company developing cell therapies for AL Amyloidosis and select immune-mediated diseases, today announced initial clinical data from the first four patients in the ongoing NEXICART-2 (NCT06097832) U.S. study of sterically-optimized BCMA-targeted chimeric antigen receptor T (CAR-T) cell therapy, NXC-201, in relapsed/refractory AL Amyloidosis. Immix plans to host a conference call to review the results on Thursday, December 19, 2024 at 11:00 AM ET.
NEXICART-2 is a Phase 1b/2, multi-site U.S. open label dose escalation and dose expansion trial enrolling relapsed/refractory AL Amyloidosis patients with preserved heart function (excluding patients with pre-existing heart failure), who have progressed on Dara-CyBorD. NEXICART-2 builds on prior ex-U.S. study NEXICART-1. NEXICART-2 has completed the first dosing cohort and is currently dosing patients at the expansion cohort dose level. NEXICART-2 is the only ongoing clinical trial of a CAR-T in the United States for AL Amyloidosis. NEXICART-2 opened for enrollment June 2024 and this is the first clinical data being reported from the study.
Immix Biopharma Announces Positive U.S. Clinical Data From First Four Patients in NEXICART-2 U.S. Trial of sterically-optimized CAR-T NXC-201 in relapsed/refractory Light Chain (AL) Amyloidosis 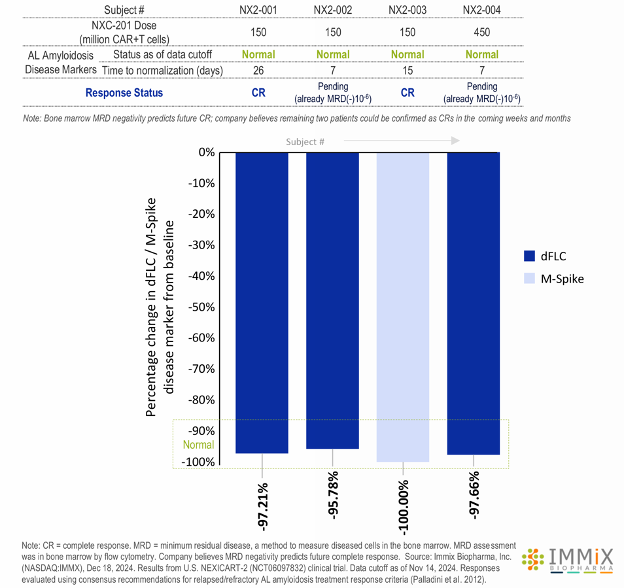
Immix Biopharma, Inc. (Nasdaq:IMMX)
“Today’s announcement represents a major step forward for Immix. We believe this first data cut of U.S. study NEXICART-2 including rapid and deep responses validates our strategy to focus on relapsed/refractory AL Amyloidosis patients with preserved heart function, who will benefit most from NXC-201 treatment,” said Ilya Rachman, M.D., Ph.D., Chief Executive Officer of Immix Biopharma. Gabriel Morris, Chief Financial Officer of Immix Biopharma, added, “We credit the resolute efforts of our investigators, sites, and team as we continue on track for NEXICART-2 interim and final read-outs.”
The results include follow-up and clinical data from four patients, and includes NX2-003 patient data through month three. Median follow-up is 85 days (range 29-141) as of the data cut-off date of November 14, 2024.
Patient Characteristics
- All patients were relapsed (n=1) or refractory (n=3) to an anti-CD38 antibody and experienced a median of 4 prior lines of therapy (range: 2-6) that failed to stop worsening of disease prior to receiving NXC-201.
- Two patients (50%) had received prior autologous stem cell transplant.
Disease Markers at enrollment
- At enrollment, median difference in free light chain (dFLC) was 65 mg/L (range: 24 -86) (among Patients NX2-001, NX2-002, and NX2-004); and Patient NX2-003 M-spike was 0.79 g/dL
- Patients were cardiac Mayo stage I/II (two Stage II, two stage I) with median NT-proBNP 389 pg/mL (range: 146 – 1,297). Three patients had New York Heart Association (NYHA) class I heart failure while one had class II
- One patient had kidney involvement with 3.0gm albuminuria in 24 hours
Clinical Results
- Three patients received 150 million and 1 received 450 million CAR+T cells
- Median follow-up was 85 days (range 29-141) as of the data cut-off date of November 14, 2024
Disease Markers after NXC-201 treatment
- All four patients treated with NXC-201 normalized their disease markers within 30 days of dosing, of which, two are already classified as complete responders (CR), and the remaining two are bone marrow MRD negative (10-6); all patients remain in response as of the data cutoff of Nov 14, 2024
- Bone marrow MRD negativity predicts future CR; company believes remaining two patients could be confirmed as CR in the coming weeks and months
-
- Patients NX2-001, NX2-002, and NX2-004 all had reduction of dFLC to <1 mg/dL, all three with minimum residual disease bone marrow (MRD)(-)(10-6) negativity in bone marrow by flow cytometry (10-6 sensitivity). Normalization of FLC occurred a median of 7 days (range: 7-26) following NXC-201 infusion.
- Patient NX2-003, enrolled based on elevated M-spike, had resolution (normalization) of the M-spike 15 days following NXC-201 (did not have elevated dFLC at enrollment).
- Improvement in NYHA class from class II to class I occurred in 1 patient 14 days following NXC-201 treatment (Patient NX2-002)
Tolerability
- No patient developed immune effector cell-associated neurotoxicity syndrome.
- No cytokine-release syndrome (CRS) was observed in two patients (patients NX2-001, NX2-002). Grade 1 (n=1), and grade 2 (n=1) CRS was observed; both onset day 3 and lasting less than 24 hours following one dose of tocilizumab (patients NX2-003 and NX2-004).
- Hematologic adverse events included neutropenia in all four patients (three grade 3, one grade 4).
- There was no febrile neutropenia, treatment-related infections, cardiac toxicity, and no deaths.
Next Steps
- Immix plans to continue enrolling patients in its potentially pivotal NEXICART-2 (NCT06097832) U.S. clinical trial for relapsed/refractory AL Amyloidosis.
- Company to provide next program update in H1 2025.
- Interim clinical data readout is expected Q2/Q3 2025.
- Final topline clinical data readout is expected Q2/Q3 2026.
Virtual Investor Event
The company will host a virtual investor event on Dec 19 at 11:00 a.m. ET: https://zoom.us/webinar/register/WN_pFUbm1i5SAiW6m5zm-0xTA .
About NEXICART-2
NEXICART-2 (NCT06097832) is an open-label, single-arm, multi-site U.S. Phase 1b/2 dose expansion clinical trial of CAR-T NXC-201 in relapsed/refractory AL Amyloidosis. NEXICART-2 is expected to enroll 40 patients with preserved heart function (excluding patients with pre-existing heart failure) who have not been exposed to prior BCMA-targeted therapy. The study is designed with a standard 6 patient safety-run in to evaluate two doses (three patients each at 150 million CAR+T cells and 450 million CAR+T cells) (both dose levels were evaluated in the NEXICART-1 study and have produced complete responses in relapsed/refractory AL Amyloidosis patients). The study aims to evaluate the safety and efficacy of NXC-201. Primary endpoints are complete response rate and overall response rate, according to consensus recommendations (Palladini et al. 2012).
About NXC-201
NXC-201 is a sterically-optimized BCMA-targeted chimeric antigen receptor T (CAR-T) cell therapy. Initial data from Phase 1b/2 ex-U.S. study NEXICART-1 has demonstrated high complete response rates and no neurotoxicity of any kind in AL Amyloidosis.
NXC-201 is being studied in a comprehensive clinical development program for the treatment of patients with relapsed/refractory AL amyloidosis in the U.S., with the potential to expand into select immune-mediated diseases. The NXC-201 NEXICART-2 (NCT06097832) U.S. clinical trial builds on a robust clinical dataset. NXC-201 has been awarded Orphan Drug Designation (ODD) in AL Amyloidosis by the US FDA and in the EU by the EMA.
About AL Amyloidosis
AL amyloidosis is caused by abnormal plasma cells in the bone marrow, which produce misfolded amyloid proteins that build-up in the heart, kidney, liver, and other organs. This build-up causes progressive and widespread damage to multiple organs, including heart failure, and leads to high mortality rates.
The U.S. observed prevalence of relapsed/refractory AL Amyloidosis is estimated to be growing at 12% per year according to Staron, et al Blood Cancer Journal, to approximately 33,277 patients in 2024.
The Amyloidosis market was $3.6 billion in 2017, and is expected to reach $6 billion in 2025, according to Grand View Research.
About Immix Biopharma, Inc.
Immix Biopharma, Inc. (ImmixBio) (Nasdaq: IMMX) is a clinical-stage biopharmaceutical company developing cell therapies for AL Amyloidosis and select immune-mediated diseases. Our lead candidate is sterically-optimized BCMA-targeted chimeric antigen receptor T (CAR-T) cell therapy NXC-201. NXC-201 is being evaluated in the U.S. Phase 1b/2 trial NEXICART-2 (NCT06097832) as well as the ex-U.S. study NEXICART-1 (NCT04720313). NXC-201 has demonstrated no neurotoxicity of any kind in AL Amyloidosis and short duration of cytokine release syndrome (CRS), supporting expansion into select immune-mediated diseases. NXC-201 has been awarded Orphan Drug Designation (ODD) in AL Amyloidosis by the US FDA and in the EU by the EMA. Learn more at www.immixbio.com and www.BeProactiveInAL.com.
Forward Looking Statements
This press release contains forward-looking statements regarding Immix Biopharma, Inc., its results of operations, prospects, future business plans and operations and the matters discussed above, including, but not limited to, the potential benefits of our product candidate CAR-T NXC-201 and the timing and results related clinical trials. These statements involve risks and uncertainties, and actual results may differ materially from any future results expressed or implied by the forward-looking statements. Forward-looking statements also include, but are not limited to, our plans, objectives, expectations and intentions and other statements that contain words such as “expects”, “contemplates”, “anticipates”, “plans”, “intends”, “believes”, “estimates”, “potential”, and variations of such words or similar expressions that convey the uncertainty of future events or outcomes, or that do not relate to historical matters. Those forward-looking statements involve known and unknown risks, uncertainties and other factors that could cause actual results to differ materially. Among those factors are: (i) the risk that the further data from the ongoing Phase 1b/2 clinical trials for CAR-T NXC-201 will not be favorably consistent with the data readouts to date, (ii) the risk that the Company may not be able to continue the NEXICART-2 multi-site U.S. Phase 1b/2 clinical trial; (iii) the risk that the Company may not be able to advance to registration-enabling studies for CAR-T NXC-201 or other product candidates, (iv) that success in early phases of pre-clinical and clinicals trials do not ensure later clinical trials will be successful; (v) that no drug product developed by the Company has received FDA pre-market approval or otherwise been incorporated into a commercial drug product, (vi) the risk that the Company may not be able to obtain additional working capital with which to continue the clinical trials for CAR-T NXC-201, or advance to the initiation of registration-enabling studies, for such product candidates as and when needed and (vii) those other risks disclosed in the section “Risk Factors” included in the Company’s Annual Report on Form 10-K filed with the SEC on March 29, 2024 and other periodic reports subsequently filed with the Securities and Exchange Commission. These reports are available at www.sec.gov. Immix Biopharma cautions that the foregoing list of important factors is not complete. Immix Biopharma cautions readers not to place undue reliance on any forward-looking statements. Immix Biopharma does not undertake, and specifically disclaims, any obligation to update or revise such statements to reflect new circumstances or unanticipated events as they occur, except as required by law. If we update one or more forward-looking statements, no inference should be drawn that we will make additional updates with respect to those or other forward-looking statements.
Contacts
Mike Moyer
LifeSci Advisors
mmoyer@lifesciadvisors.com
Company Contact
irteam@immixbio.com
Attachment





