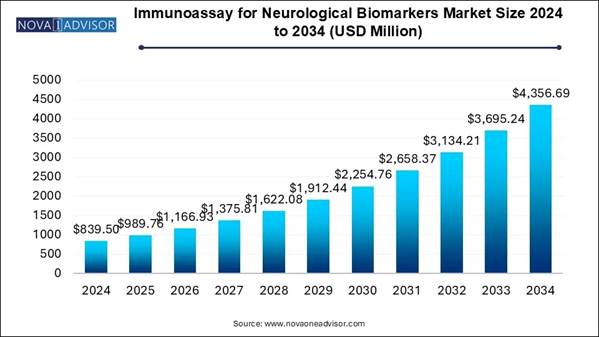
The global immunoassay for neurological biomarkers market size was valued at USD 839.5 million in 2024 and is projected to hit USD 4356.69 million by 2034, registering a CAGR of 17.9% from 2025 to 2034. Increasing expenditure in the R&D of treatments for neurological diseases is the key factor driving market growth. Also, the growing prevalence of neurological diseases coupled with the ongoing government initiatives can fuel market growth further.
Immunoassay for Neurological Biomarkers Market Key Takeaways:
· The reagents segment held the largest share of 66.78% in 2024.
· The instruments segment is anticipated at a CAGR of 17.6% during the forecast period.
· The Alzheimer’s disease segment held the largest share of 38.04% in 2024.
· The Alzheimer’s disease segment is expected to expand at the highest CAGR of 18.4% during the forecast period.
· The research application segment captured the highest revenue share of 69.0% in 2024.
· in vitro diagnostics application segment is anticipated to expand at rapid pace with a CAGR of 23.9% during the forecast period.
· North America held the largest share of 49.0% in the immunoassay for neurological biomarkers market in 2024.
· Asia Pacific is estimated to expand at the fastest CAGR of 18.6% from 2025-2034.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report@ https://www.novaoneadvisor.com/report/sample/7872
The immunoassay for neurological biomarkers market is witnessing a major growth contributed by the increasing demand for neuro immunoassay for pharmaceutical analysis like diagnosis of disease, pharmacokinetic, and bioequivalence of drug discovery. The market growth is also expected to be boosted by a rising introduction and approval of immunoassay instruments. In addition, technological advancements in the field can strengthen market expansion further.
One of the major opportunities driving the growth of the market is the innovations in biomarker research to know the pathophysiology of this disorder. The market opportunities lie in overcoming hurdles such as ethical responsibilities, laboratory errors, high cost of analyses, and normal range difficulty to start.
The world of clinical research, especially biomarker-led trials, is experiencing an AI transformation. The volume of data and sheer complexity in these trials call for technological advancement. Furthermore, AI technologies with the ability to process large amounts of data, identify potential biomarkers, and even predict patient responses. AI-driven tools demonstrate tangible enhancements in oncology clinical trials.
Market Trends
· Innovations in Biotechnology and Genomic Research: Innovations in proteomics, genomics, and molecular biology have contributed to the development of far more precise and accurate biomarkers for neurologic disorders. These innovations have improved diagnostic precision and optimized the timely detection of diseases, leading to market growth shortly.
· Rising Demand for Personalized Medicine: Personalized medicine is increasingly getting over other pharmaceutical elements, in which treatment methods and interventions are personalized and formulated as per the requirement of each patient, depending on their biomarkers and genetic makeup.
· Extensive Investments in R&D: There is an increasing number of investments and research done on neuroscience research. This has impelled some healthcare and medical giants and governments to facilitate sanctions towards development and numerous research activities, hence propelling the market growth over the forecast period.
Immunoassay for Neurological Biomarkers Market Report Scope
|
Report Attribute |
Details |
|
Market size value in 2025 |
USD 989.76 Million |
|
Revenue forecast in 2034 |
USD 4356.69 Million |
|
Growth rate |
CAGR of 17.9% from 2025 to 2035 |
|
Base year for estimation |
2024 |
|
Historical data |
2018 - 2024 |
|
Forecast period |
2025 - 2034 |
|
Quantitative units |
Revenue in USD million, CAGR from 2025 to 2034 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, trends |
|
Segments covered |
Product, Disease, Application, Region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
|
Country scope |
U.S.; Canada; U.K.; Germany; France; Italy; Spain; Denmark; Sweden; Norway; Japan; China; India; Australia; Thailand; South Korea; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait |
|
Key companies profiled |
QIAGEN; Abbott; Merck & Co., Inc.; Johnson & Johnson Services, Inc.; Thermo Fisher Scientific, Inc.; Bio-Rad Laboratories, Inc.; Sysmex Corporation; Merck KGaA; F. Hoffmann La-Roche Ltd.; Nimble Therapeutics |
|
Customization scope |
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope. |
Segment Insights
By Product Insights
The reagents segment held the largest share of 66.78% in 2024. The dominance of the segment can be attributed to the ongoing product approvals and launches of novel immunoassay reagents. The market is also experiencing a shift from conventional reagents to ultrasensitive reagents, which can impact segment growth positively further.
The instruments segment is anticipated at a CAGR of 17.6% during the forecast period. The growth of the segment can be linked to the rising launch and approval of immunoassay instruments by the market players. Additionally, these instruments offer features such as high throughput and the capability to run multiple tests simultaneously, which makes them crucial for large-scale research and diagnostic applications.
By Disease Insights
The Alzheimer’s disease segment held the largest share of 38.04% in 2024. The dominance of the segment can be credited to the increased incidence of Alzheimer's disease along with the growing need for early diagnosis and efficient treatment management. Alzheimer's poses a crucial medical challenge across the globe, with a sudden surge in the elderly population boosting its escalating incidence.
The Alzheimer’s disease segment is expected to expand at the highest CAGR of 18.4% during the forecast period. The growth of the segment can be driven by the increasing prevalence of this disease among the majority of the population across the globe. Parkinson's disease is a neurodegenerative condition of neurons that creates dopamine. This neurological loss results in motor disturbances such as muscular rigidity, postural instability, and resting tremors.
By Application Insights
The research application segment captured the highest revenue share of 69.0% in 2024. The dominance of the segment is due to the important role of Immunoassays in neurological R&D. It is increasingly being used to measure biomarkers and to determine pharmacokinetics of biologics. Also, researchers are involved in detecting key biomarkers in plasma and serum to develop assays that are sensitive in considering low levels of biomarkers.

Furthermore, in vitro diagnostics application segment is anticipated to expand at rapid pace with a CAGR of 23.9% during the forecast period. The growth of the segment is because of an increasing number of disease-modifying treatments for neurological conditions. Moreover, the government is taking numerous initiatives to offer better facilities, like reimbursement policies for these tests.
By Regional Insights
Increased Healthcare Expenditure: North America to Sustain as a Leader
North America held the largest share of 49.0% in the immunoassay for neurological biomarkers market in 2024. The dominance of the region can be attributed to the surge in healthcare expenditure along with the increase in customer awareness regarding the use of biomarkers in various disorders. However, the wide availability of technologically innovative products and ongoing government initiatives can impact market growth positively. Major market players in the region are emphasizing new product launches for the detection of neurological biomarkers.

The U.S. Immunoassay for Neurological Biomarkers Market Trends
The US led the market in 2024. The market growth in the U.S. is credited to the increasing incidence of neurological diseases and the rising number of clinical trials in the region.
· In July 2024, TOKYO & MALVERN, Pa. & GENT, Belgium H.U. Group Holdings Inc., and its wholly owned subsidiary Fujirebio announced the availability of the Lumipulse G GFAP assay for the fully automated, random-access LUMIPULSE® G immunoassay systems. The Research Use Only (RUO) assay is now available in the United States, and it will be available in Japan, Europe, and other regions* as of September this year.
The surge in Geriatric Population: Asian Countries to Boom
Asia Pacific is expected to grow at the fastest rate over the forecast period. The growth of the region can be linked to the increasing elderly population which is more prone to these neurological disorders coupled with the growing incidence of neurological diseases in developing countries such as China and India. Furthermore, the rising need for timely diagnosis of neurological diseases to decrease mortality & treatment costs can propel market growth in the region soon.
Immediate Delivery Available | Buy This Premium Research https://www.novaoneadvisor.com/report/checkout/7872
· Automated Immunoassay Analyzers Market - https://www.precedenceresearch.com/automated-immunoassay-analyzers-market
· Cardiac Biomarkers Market- https://www.precedenceresearch.com/cardiac-biomarkers-market
· Chemiluminescence Immunoassay Market - https://www.precedenceresearch.com/chemiluminescence-immunoassay-market
· Biomarkers Market - https://www.precedenceresearch.com/biomarkers-market
· Digital Biomarkers Market- https://www.precedenceresearch.com/digital-biomarkers-market
· Cancer Biomarkers Market- https://www.precedenceresearch.com/cancer-biomarkers-market
Immunoassay for Neurological Biomarkers Market Top Companies
· QIAGEN
· Abbott
· Johnson & Johnson Services, Inc.
· Thermo Fisher Scientific, Inc.
· Bio-Rad Laboratories, Inc.
· Merck KGaA
· Nimble Therapeutics
Immunoassay for Neurological Biomarkers Market Recent Developments
· In July 2024, Researchers from IIT-BHU, Varanasi, India, developed the 'lab on a chip' to make early, simple detection of neurological conditions feasible. This system would be an improvement over more dependable and fast diagnoses of such diseases as schizophrenia, Parkinson's disease, or depression.
· In March 2024, ETNA-MS, or Eye-Tracking Neurological Assessment for Multiple Sclerosis, was also cleared by Health Canada for its use in following disease progression among multiple sclerosis (MS) patients.
· In October 2023, C2N Diagnostics has developed a state-of-the-art fluid biomarker for Alzheimer's disease. This new test is designed to help researchers track neurofibrillary "Tau" tangle pathology, a significant step forward in the understanding and monitoring of Alzheimer's progression through the identification of Tau protein abnormalities.
· In January 2023, the United States FDA granted approval for Leqembi (lecanemab-irmb). The approval was obtained through the Accelerated Approval pathway. It belongs to a new category of medications that target the fundamental pathophysiology of Alzheimer's disease. This approval marks a significant advancement in the ongoing efforts to effectively treat Alzheimer's disease.
Segment Covered in the Report
By Product
· Instruments
· Reagents
· Services
By Disease
· Alzheimer's Disease
· Parkinson's Disease
· Multiple Sclerosis
· Others
By Application
· In Vitro Diagnostics Application
· Research Application
By Regional
· North America
· Europe
· Asia Pacific
· Latin America
· Middle East and Africa (MEA)
Immediate Delivery Available | Buy This Premium Research https://www.novaoneadvisor.com/report/checkout/7872
USA: +1 804 441 9344
APAC: +61 485 981 310 or +91 87933 22019
Europe: +44 7383 092 044
Email: sales@novaoneadvisor.com
Web: https://www.novaoneadvisor.com/
You can place an order or ask any questions, please feel free to contact at sales@novaoneadvisor.com | +1 804 441 9344
