DANVERS, Mass., Oct. 28, 2024 /PRNewswire/ -- Results were presented at Transcatheter Cardiovascular Therapeutics (TCT) 2024 conference from the first completed pivotal trial on patients supported with Impella ECP™, a novel transvalvular axial flow pump with compressible pump architecture. Impella ECP is a technology of Abiomed, part of Johnson & Johnson MedTech, and the global leader in heart recovery.
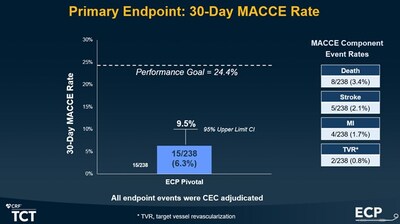
The pivotal investigational device exemption (IDE) study, which enrolled 256 patients at 18 sites in the US, met the primary endpoint. MACCE (Major Adverse Cardiac and Cerebrovascular Events) rate at 30 days was 6.3%, significantly below the pre-defined performance goal (see figure 1). The study demonstrated Impella ECP's safety and efficacy for use in high-risk PCI. Operators chose 8Fr Angio-Seal as the first-closure method in 70% of the patients, with a 92% success rate1. (See full presentation)
The study was led by Principal Investigator Amir Kaki, MD2, Director of Mechanical Circulatory Support and Complex Coronary Intervention at Henry Ford - St. John Hospital and Medical Center. "The study met its prespecified 30-day primary endpoint with low complication rates and the 9Fr arterial access enabled a high success rate closing with an 8Fr Angio-Seal," said Dr. Kaki. "Impella ECP technology with small-bore access and closure offers benefits for patients and physicians."
With a 9Fr size at insertion, Impella ECP is designed to be implanted and removed using small bore access and closure techniques. After insertion, Impella ECP expands to 21Fr to provide circulatory support and left ventricular (LV) unloading for high-risk PCI.
Impella ECP is an investigational device limited by federal law to investigational use only. Impella ECP is in use as part of the FDA-approved continuous access program at selected US sites. As a next step, Impella ECP will be submitted to the US FDA for approval.
About Cardiovascular Solutions from Johnson & Johnson MedTech
Across Johnson & Johnson, we are tackling the world's most complex and pervasive health challenges. Through a cardiovascular portfolio that provides healthcare professionals with advanced mapping and navigation, miniaturized tech, and precise ablation, we are addressing conditions with significant unmet needs such as heart failure, coronary artery disease, stroke, and atrial fibrillation. We are the global leaders in heart recovery, circulatory restoration and the treatment of heart rhythm disorders, as well as an emerging leader in neurovascular care, committed to taking on two of the leading causes of death worldwide in heart failure and stroke. For more information about heart recovery technology, visit www.heartrecovery.com.
About Johnson & Johnson
At Johnson & Johnson, we believe health is everything. Our strength in healthcare innovation empowers us to build a world where complex diseases are prevented, treated, and cured, where treatments are smarter and less invasive, and solutions are personal. Through our expertise in Innovative Medicine and MedTech, we are uniquely positioned to innovate across the full spectrum of healthcare solutions today to deliver the breakthroughs of tomorrow, and profoundly impact health for humanity. Learn more about our MedTech sector's global scale and deep expertise in cardiovascular, orthopaedics, surgery and vision solutions at https://thenext.jnjmedtech.com. Follow us @JNJMedTech and on LinkedIn. Abiomed, Inc. is a Johnson & Johnson MedTech company.
Cautions Concerning Forward-Looking Statements
This press release contains "forward-looking statements" as defined in the Private Securities Litigation Reform Act of 1995 regarding the Impella Platform. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Abiomed, Inc. and/or Johnson & Johnson. Risks and uncertainties include, but are not limited to: uncertainty of regulatory approvals; uncertainty of commercial success; challenges to patents; competition, including technological advances, new products and patents attained by competitors; product efficacy or safety concerns resulting in product recalls or regulatory action; changes to applicable laws and regulations, including global health care reforms; changes in behavior and spending patterns of purchasers of healthcare products and services; and trends toward healthcare cost containment. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson's Annual Report on Form 10-K for the fiscal year ended December 31, 2023, including in the sections captioned "Cautionary Note Regarding Forward-Looking Statements" and "Item 1A. Risk Factors," and in Johnson & Johnson's subsequent Quarterly Reports on Form 10-Q and other filings with the Securities and Exchange Commission. Copies of these filings are available online at sec.gov, jnj.com or on request from Johnson & Johnson. Neither Abiomed, Inc. nor Johnson & Johnson undertakes to update any forward-looking statement as a result of new information or future events or developments.
1 Success defined as hemostasis with first and only device and no additional closure strategy.
2 Dr. Amir Kaki is a paid consultant and speaker for Abiomed, Inc. He was not paid for his quote in this press release.
Contact: Jenny Leary, jleary1@its.jnj.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/impella-ecp-pivotal-study-demonstrates-safety-efficacy-for-use-in-high-risk-pci-302288506.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/impella-ecp-pivotal-study-demonstrates-safety-efficacy-for-use-in-high-risk-pci-302288506.html
SOURCE Johnson & Johnson MedTech