LAG 3 Next Generation Immunotherapy Market Outlook 2025-2035:
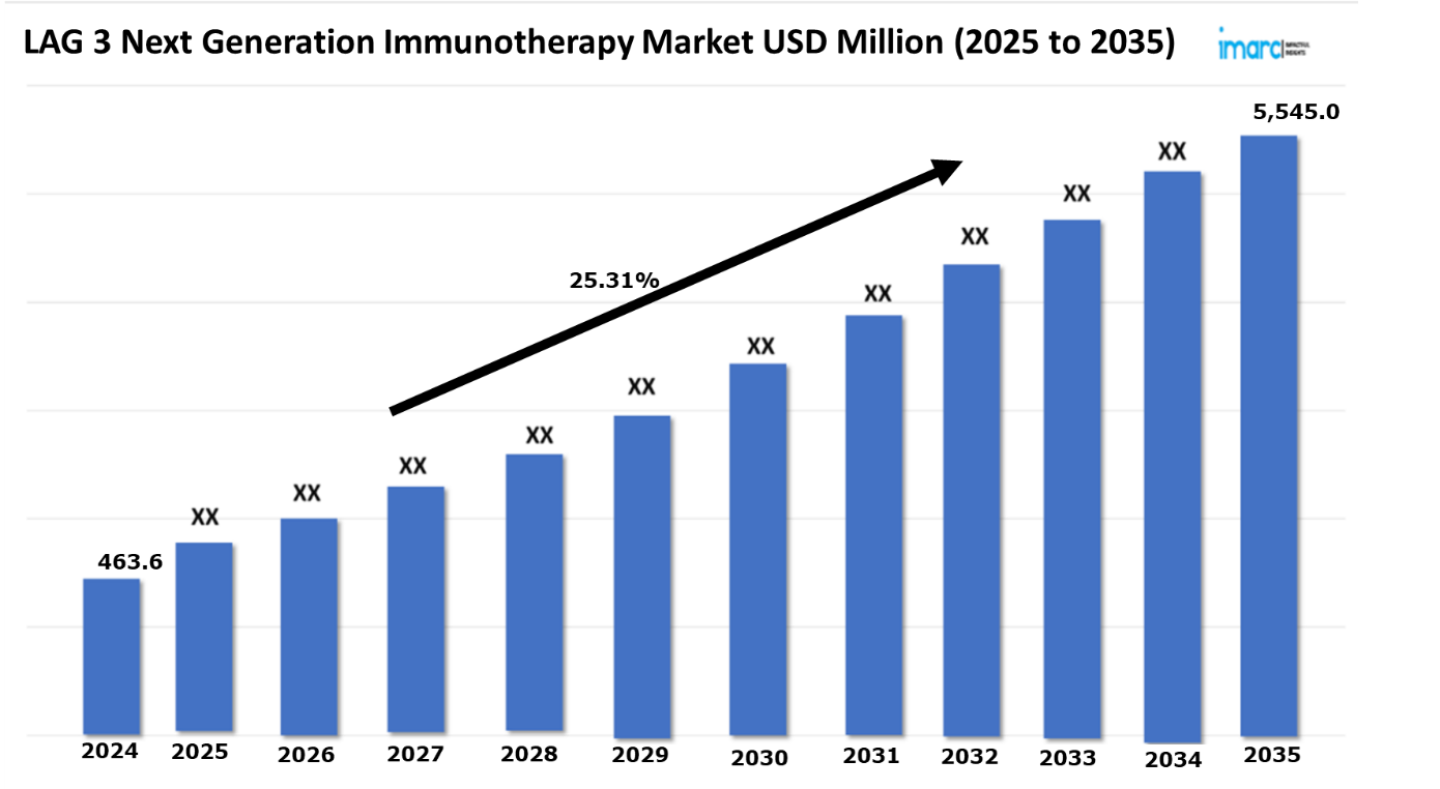
The Lag-3 Next Generation Immunotherapy market reached a value of USD 463.6 Million in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 5,545.0 Million by 2035, exhibiting a growth rate (CAGR) of 25.31% during 2025-2035. The treatment landscape for LAG-3, or Lymphocyte-Activation Gene 3, next-generation immunotherapy is undergoing a radical shift from the traditional immune checkpoint inhibitors to more advanced and targeted treatments that support anti-tumor immunity. The driving force behind this paradigm shift is largely our increasing understanding of T-cell exhaustion mechanisms and our recognition of LAG-3’s critical role in immune regulation. As a result, there have been remarkable discoveries of new checkpoint inhibitors, besides the unprecedented advances in combination immunotherapies. In the course of this revolution, there is now FDA approval for Nivolumab/relatlimab (Opdualag) which was announced as the first LAG-3 inhibitor that is being launched. Results of clinical studies also show that in combination with PD-1 blockade, relatlimab provides significantly enhanced antitumor efficacy and has shown a potential for great improvement of patients’ conditions when treated with the disease. Also, the landscape of treatment continues to grow with the incorporation of bispecific antibodies, dual checkpoint blockade strategies, and individualized biomarker-driven therapies. These new approaches synergistically combine LAG-3 inhibition with established PD-1/PD-L1 therapies to aim for the best immune activation strategy that will potentially help in surmounting resistance issues many patients encounter.
Advancements in Biomarker-Driven Precision Medicine in LAG-3 Immunotherapy
The integration of LAG-3 biomarkers in clinical trials is revolutionizing the development of next-generation immunotherapies, allowing for a more precise and personalized approach to cancer treatment. Researchers can predict who will respond best to LAG-3 inhibitors by identifying patients with high LAG-3 expression, thus optimizing treatment selection and improving clinical outcomes. This biomarker-directed approach aims to more finely stratify patients, minimize exposure to ineffective treatments, and enhance the effect of therapy. Another aspect is that companion diagnostics are been incorporated in trials to accelerate the identification of subgroups defined by biomarkers, which ultimately translates to more effective therapies targeted precisely on effective subgroups. Together with LAG-3 expression profiling, other immune checkpoint markers, such as PD-1/PD-L1, are further refining dual checkpoint blockade strategies to maximize synergy in immunotherapy. Further, biomarker-based precision medicine can lead to more effective and individualized cancer treatments as research progresses along with the development of liquid biopsies and AI-driven predictive models.
Request a PDF Sample Report:
https://www.imarcgroup.com/lag-3-next-generation-immunotherapy-market/requestsample
Regulatory Support & Fast-Track Designations for LAG-3 Immunotherapy
The FDA in the United States and the European Medicines Agency (EMA) are driving innovation in immuno-oncology by moving forward proactively to promote the development of LAG-3 inhibitors. Such agencies embrace mechanisms such as Fast Track, Breakthrough Therapy, and Orphan Drug designations to speed up the development of promising candidates. These approvals both compress timelines towards market but equally and importantly accelerate patients’ earlier access to ground-breaking immunotherapies while unlocking quicker progress in the market through clinical studies. One historic illustration is how approval of Opdualag - Nivolumab/relatlimab- laid the great significance and paved precedential standards on all LAG-3 targeted treatment products thus far, by prompting more and much-needed investment that promotes strengthened collaboration with these drugs from a regulatory point of view.
Buy Full Report:
https://www.imarcgroup.com/checkout?id=6768&method=809
Marketed Therapies in the LAG 3 Next Generation Immunotherapy Market
Opdualag (Nivolumab/relatlimab) - Bristol-Myers Squibb
LAG-3 next-generation immunotherapy represents a significant advancement in cancer treatment by enhancing T-cell activation and overcoming immune exhaustion. Opdualag (relatlimab + nivolumab), developed by Bristol-Myers Squibb, is the first FDA-approved LAG-3 inhibitor, demonstrating improved efficacy in treating unresectable or metastatic melanoma.
Leading Companies in the LAG 3 Next Generation Immunotherapy Market:
The treatment landscape for LAG-3 next-generation immunotherapy is undergoing a significant transformation, driven by intense competition and continuous innovation within the biotechnology and pharmaceutical sectors. Leading companies such as Bristol-Myers Squibb is at the forefront, investing heavily in the development of LAG-3 inhibitors and combination immunotherapies. This dedication is reflected in the pursuit of groundbreaking approaches, including Opdualag (relatlimab + nivolumab), the first FDA-approved LAG-3 checkpoint inhibitor, which has demonstrated enhanced efficacy in metastatic melanoma. The growing focus on dual checkpoint blockade strategies, bispecific antibodies, and biomarker-driven treatments underscores a collective effort to provide more effective and personalized cancer immunotherapies. These advancements aim not only to enhance T-cell activation and overcome immune resistance but also to significantly improve survival rates and quality of life for patients with advanced and immunotherapy-resistant cancers.
Key Players in the LAG 3 Next Generation Immunotherapy Market:
The key players in the LAG 3 Next Generation Immunotherapy market who are in different phases of developing different therapies are Bristol-Myers Squibb and others.
Regional Analysis:
The treatment landscape for LAG-3 next-generation immunotherapy is undergoing a significant transformation, propelled by cutting-edge advancements in immune checkpoint inhibitors and combative immunotherapies. Surgeons of this evolution are predominantly located in major biotech and pharmaceutical centers across the United States and Europe, with the U.S. emerging as a leader due to substantial research funding and the increasing adoption of immune-oncology therapies. At the heart of this progress is a paradigm shift from traditional single-agent checkpoint inhibitors to sophisticated dual blockade and combination therapies, which aim to amplify T-cell activation and surmount barriers associated with immune resistance. Notably, LAG-3 inhibitors, such as Opdualag — a combination of relatlimab and nivolumab — have shown remarkable effectiveness in the treatment of metastatic melanoma, illuminating a new path for managing challenging cancers. As the treatment approach for LAG-3-based immunotherapy matures, there is an intensified focus on personalized medicine, emphasizing biomarker-driven strategies and the development of bispecific antibodies. These targeted approaches are designed to tailor therapies to individual patients, thereby optimizing therapeutic efficacy and enhancing patient outcomes.
Recent Developments in the LAG 3 Next Generation Immunotherapy Market:
· In April 2022, The Food and Drug Administration (FDA) approved a combination immunotherapy for the treatment of certain patients with advanced melanoma. This treatment combines relatlimab and nivolumab (Opdivo) and will be commercially available under the name Opdualag.
Key information covered in the report.
Base Year: 2024
Historical Period: 2019-2024
Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the LAG 3 Next Generation Immunotherapy market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the LAG 3 Next Generation Immunotherapy market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current LAG 3 Next Generation Immunotherapy-marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC:
https://www.imarcgroup.com/lag-3-next-generation-immunotherapy-market/toc
IMARC Group Offer Other Reports:
Hyperhidrosis Market: The 7 major hyperhidrosis market reached a value of US$ 426.7 Million in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 651.2 Million by 2034, exhibiting a growth rate (CAGR) of 3.92% during 2024-2034.
Food Allergy Market: The 7 major food allergy market reached a value of US$ 2,199.4 Million in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 3,425.2 Million by 2034, exhibiting a growth rate (CAGR) of 4.11% during 2024-2034.
Maple Syrup Urine Disease Market: The 7 major maple syrup urine disease market are expected to exhibit a CAGR of 11.2% during 2024-2034.
Cutaneous T-Cell Lymphoma Market: The 7 major Cutaneous T-cell-lymphoma market reached a value of US$ 428.2 Million in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 628.7 Million by 2034, exhibiting a growth rate (CAGR) of 3.55% during 2024-2034.
Metabolic Acidosis Market: The 7 major Metabolic acidosis market are expected to exhibit a CAGR of 5.76% during 2024-2034.
Angioedema Market: The 7 major Angioedema market are expected to exhibit a CAGR of 7.01% during 2025-2035.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800