Leukocyte Disorders Market Outlook 2025-2035:
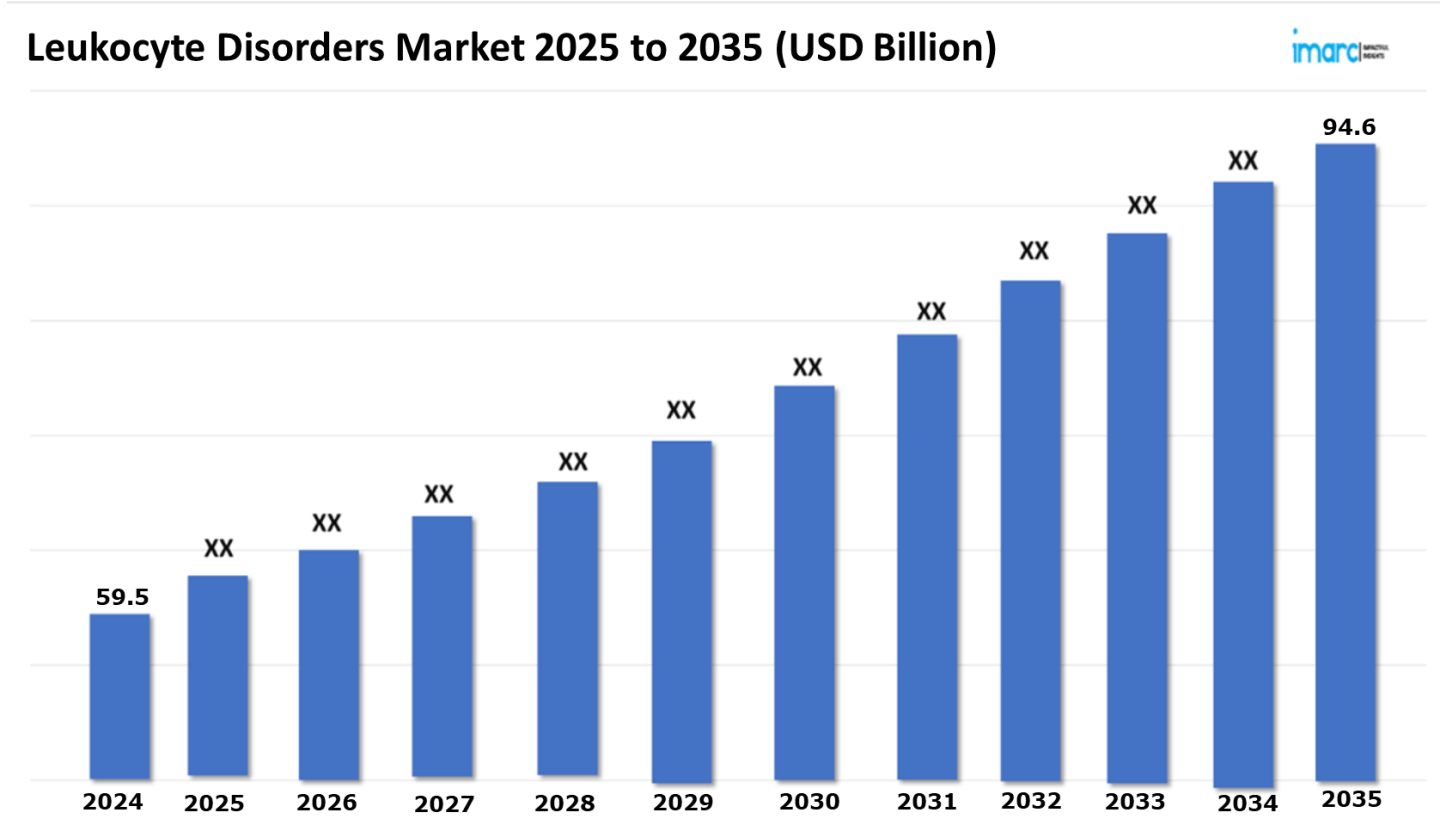
The 7 major leukocyte disorders market reached a value of USD 59.5 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 94.6 Billion by 2035, exhibiting a growth rate (CAGR) of 4.32% during 2025-2035. The growing adoption of advanced non-invasive as well as minimally invasive treatment options drives the Leukocyte Disorders market. These therapies, such as gene therapy, monoclonal antibody treatments, and cell-based therapies, effectively manage the symptoms of rare immune disorders while minimizing discomfort and recovery time. These treatments have proven particularly beneficial for diseases such as Leukocyte Adhesion Deficiency (LAD), Chronic Granulomatous Disease (CGD), and Severe Combined Immunodeficiency (SCID) by enhancing the immune functions of the patients, decreasing the rate of infections, and consequently improving the overall quality of life of the patient. Targeted biologics, which specifically modulate a pathway in the immune system, have resulted in improved patient outcomes with fewer adverse effects, which further improves the treatment satisfaction level. These cutting-edge treatments make patients and healthcare providers less dependent on long-term systemic medication and on invasive, intrusive measures, and are thus preferred as options. As these new treatments are evolving, the efficiency, personalization, and convenience offered by them when treating leukocyte disorders continue to improve.
Advances in Early Detection and Diagnostic Technologies: Driving the Leukocyte Disorders Market
Modern diagnostic and treatment technologies are revolutionizing the Leukocyte Disorders market. Management and outcomes in patients have dramatically improved, and new expertise, such as genetic testing or next-generation sequencing (NGS), as well as high-resolution imaging, enable the specific identification of mutations or abnormalities in genes controlling immune function. These innovations allow for early diagnosis and allow healthcare providers to create personalized treatment plans based on the genetic and immunological profile of the patient, thus having more accurate and effective interventions. Artificial intelligence in diagnostics further provides precision by automating the classification of genetic mutations, monitoring disease progression, and predicting responses to treatment. AI-based tools can make the identification of leukocyte disorders easier and help in the management of complex conditions by enhancing decision-making and reducing dependence on subjective assessments. Non-invasive therapies, including gene therapies, monoclonal antibody treatments, and cell-based therapies, have emerged as promising options for managing leukocyte disorders such as Leukocyte Adhesion Deficiency (LAD) and Chronic Granulomatous Disease (CGD). Such therapies focus on specific action, attacking the root causes of immune dysfunction while avoiding potential side effects and reducing long-term detrimental effects. Wearable technologies that can monitor the activity of immune cells and real-time biomarkers provide continuous monitoring of the patients, helping in timely intervention and thereby making care much better.
Request a PDF Sample Report: https://www.imarcgroup.com/leukocyte-disorders-market/requestsample
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The novel therapies coupled with advanced pharmacological treatments are the driving force in the development of the Leukocyte Disorders market. New therapies, such as targeted biologics and small molecules, are being developed to treat root immune dysfunctions due to leukocyte disorders. Such novel therapies provide many benefits including better effectiveness, few side effects, and specific mechanisms of action, hence leading to better clinical outcomes and patient satisfaction. There is also intense research on biological drugs in order to help control moderate-to-severe cases of LAD and other chronic inflammatory diseases. Some biological agents such as monoclonal antibodies target pro-inflammatory cytokines, like interleukin-1 and interleukin-17. This is pivotal for modulating the immune responses to reduce inflammation as well as its microbial and immunological cause: leukocyte dysfunction. Advances in drug delivery systems, including liposomal formulations, nanotechnology-based carriers, and hydrogels, allow for highly localized drug delivery to the specific site of immune dysregulation. This results in a higher concentration of the therapeutic at the site of action, with less systemic exposure and fewer side effects. Adjunctive therapies to restore immune function are also being explored. Examples include probiotics and immunomodulators as emerging treatments to restore microorganisms and help the immune system recover itself. Combination treatments combining the anti-inflammatory, antimicrobial and biologicals classes of drugs have more recently been discovered to be effective in treating leukocyte disorders, which presents a complex pathophysiology. Currently, there is high interest in easy-to-apply, non-invasive solutions, such as gene therapy and disruption agents for biofilm, in order to decrease the burden of invasive therapy. Innovations are also focusing more on patient-oriented care, making treatment strategies less invasive or cumbersome while giving the best, and finding the most efficient forms of long-term management of disorders caused by leukocyte dysfunction.
Buy Full Report: https://www.imarcgroup.com/checkout?id=12346&method=809
Marketed Therapies in Leukocyte Disorders Market
Neupogen (Filgrastim): Amgen/Roche
Neupogen (Filgrastim) is a recombinant granulocyte colony-stimulating factor (G-CSF) used to stimulate the production of neutrophils in patients with leukocyte disorders, particularly in those with neutropenia, a condition often seen in leukocyte adhesion deficiency (LAD) and other immune deficiencies. It helps reduce the risk of infections by boosting white blood cell counts and improving immune function.
Nyvepria (Pegfilgrastim biosimilar): Pfizer
Nyvepria is a biosimilar to Neulasta (pegfilgrastim) used in the treatment of leukocyte disorders, particularly to reduce the risk of infection in patients undergoing chemotherapy. It works by stimulating the production of white blood cells, specifically neutrophils, to enhance immune function and help prevent infections, which are common in individuals with neutropenia caused by cancer treatments.
Ryzneuta (Efbemalenograstim alfa): Evive Biotech
Ryzneuta is a novel drug being developed for the treatment of leukocyte disorders, particularly Leukocyte Adhesion Deficiency (LAD). It aims to improve neutrophil function by targeting key immune pathways, potentially enhancing immune response and reducing recurrent infections in affected patients. The drug is undergoing clinical trials to evaluate its safety and efficacy in treating chronic immune-related conditions.
Emerging Therapies in Leukocyte Disorders Market
Mavorixafor: X4 Pharmaceuticals
Mavorixafor is an investigational drug being developed for the treatment of leukocyte disorders, specifically WHIM syndrome, a rare genetic disorder caused by mutations in the CXCR4 gene. By targeting and modulating the CXCR4 receptor, Mavorixafor is designed to enhance neutrophil mobilization and improve immune cell function, aiming to reduce infections and other complications associated with impaired leukocyte adhesion and trafficking in affected patients.
Drug Name | Company Name | MOA | ROA |
Mavorixafor | X4 Pharmaceuticals | CXCR4 receptor antagonists | Oral |
Detailed list of emerging therapies in Leukocyte Disorders is provided in the final report…
Leading Companies in the Leukocyte Disorders Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global Leukocyte Disorders market, several leading companies are at the forefront of developing integrated platforms to enhance the management of Leukocyte Disorders. Some of the major players include Amgen, Roche, Pfizer, Evive Biotech, X4 Pharmaceuticals, and others. These companies are driving innovation in the Leukocyte Disorders market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for Leukocyte Disorders.
In November 2023, The U.S. Food and Drug Administration (FDA) has approved Ryzneuta (efbemalenograstim alfa-vuxw), a leukocyte growth factor, to reduce the occurrence of infections, specifically febrile neutropenia, in adult patients with non-myeloid cancers who are undergoing myelosuppressive chemotherapy that carries a significant risk of febrile neutropenia.
Key Players in Leukocyte Disorders Market:
The key players in the Leukocyte Disorders market who are in different phases of developing different therapies are Amgen, Roche, Pfizer, Evive Biotech, X4 Pharmaceuticals, and Others.
Regional Analysis:
The main markets for Leukocyte Disorders include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. As estimated by IMARC, the patient pool for Leukocyte Disorders is largest in the United States, while at the same time, it is also the largest market for treating Leukocyte Disorders. With recent developments in the treatment of Leukocyte Disorders, new therapies include advanced leukocyte-modulating agents, antimicrobial peptides, and immune system enhancers. This approach has the root causes of these disorders directly targeted for remediation. As such, newer therapies seek to restore function of the immune system and resolve chronic inflammatory conditions with improved outcomes in patients. The new strategies for treatment focus on modulating immune cell behavior by targeting cytokines like interleukin-1 and interleukin-6 and improving leukocyte adhesion and migration.
Modern diagnostic techniques, including molecular profiling, imaging, and AI-driven tools, have significantly enhanced the ability to detect and monitor leukocyte disorders more accurately. Earlier diagnosis allows for timely intervention and more focused treatments, which can greatly improve patient outcomes and reduce complications. Investment in research and development and collaboration between pharmaceutical companies, diagnostic innovators, and the research institutions are also compelling forces driving the growth of the Leukocyte Disorders market. As a result, there are numerous promising therapeutic candidates, including biologics and cell-based therapies, that are entering clinical stages. In addition, telemedicine platforms and AI-based diagnostic tools provide access to care, ensuring the timely treatment of patients in remote or underserved regions. This shift is helping democratize healthcare, while regions like North America and Europe continue to lead the way in advancing treatment options, contributing to sustained global market growth.
Recent Developments in Leukocyte Disorders Market:
· In November 2024, X4 Pharmaceuticals announced encouraging new clinical results from its completed Phase 2 trial of mavorixafor, an oral CXCR4 antagonist, for the treatment of chronic neutropenia (CN). Final data analysis from the six-month study demonstrated that once-daily oral mavorixafor significantly and sustainably increased participants’ mean absolute neutrophil counts (ANC). Furthermore, when used in combination with injectable granulocyte colony-stimulating factor (G-CSF), mavorixafor treatment allowed for a substantial reduction in G-CSF dosage while still maintaining normal mean ANC levels.
Key information covered in the report.
Base Year: 2024
Historical Period: 2019-2024
Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the Leukocyte Disorders market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the Leukocyte Disorders market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current Leukocyte Disorders marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC: https://www.imarcgroup.com/leukocyte-disorders-market/toc
IMARC Group Offer Other Reports:
Human Immunodeficiency Virus Type 1 Market: The 7 major human immunodeficiency virus type 1 market reached a value of US$ 24.4 Billion in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 39.8 Billion by 2034, exhibiting a growth rate (CAGR) of 4.56% during 2024-2034.
Cone Rod Dystrophy Market: The 7 major cone rod dystrophy market reached a value of US$ 114.5 Million in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 179.0 Million by 2034, exhibiting a growth rate (CAGR) of 4.15% during 2024-2034.
Granulomatous Disease Market: The 7 major granulomatous disease market are expected to exhibit a CAGR of 4.6% during 2024-2034.
Agammaglobulinemia Market: The 7 major agammaglobulinemia market are expected to exhibit a CAGR of 6.9% during 2024-2034.
Febrile Neutropenia Market: The 7 major febrile neutropenia market reached a value of US$ 10.4 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach US$ 16.3 Billion by 2034, exhibiting a growth rate (CAGR) of 4.15% during 2024-2034.
Chemotherapy-Induced Neutropenia Market: The 7 major chemotherapy-induced neutropenia market reached a value of US$ 371.2 Million in 2023. Looking forward, IMARC Group expects the 7MM to reach US$ 455.7 Million by 2034, exhibiting a growth rate (CAGR) of 1.88% during 2024-2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800