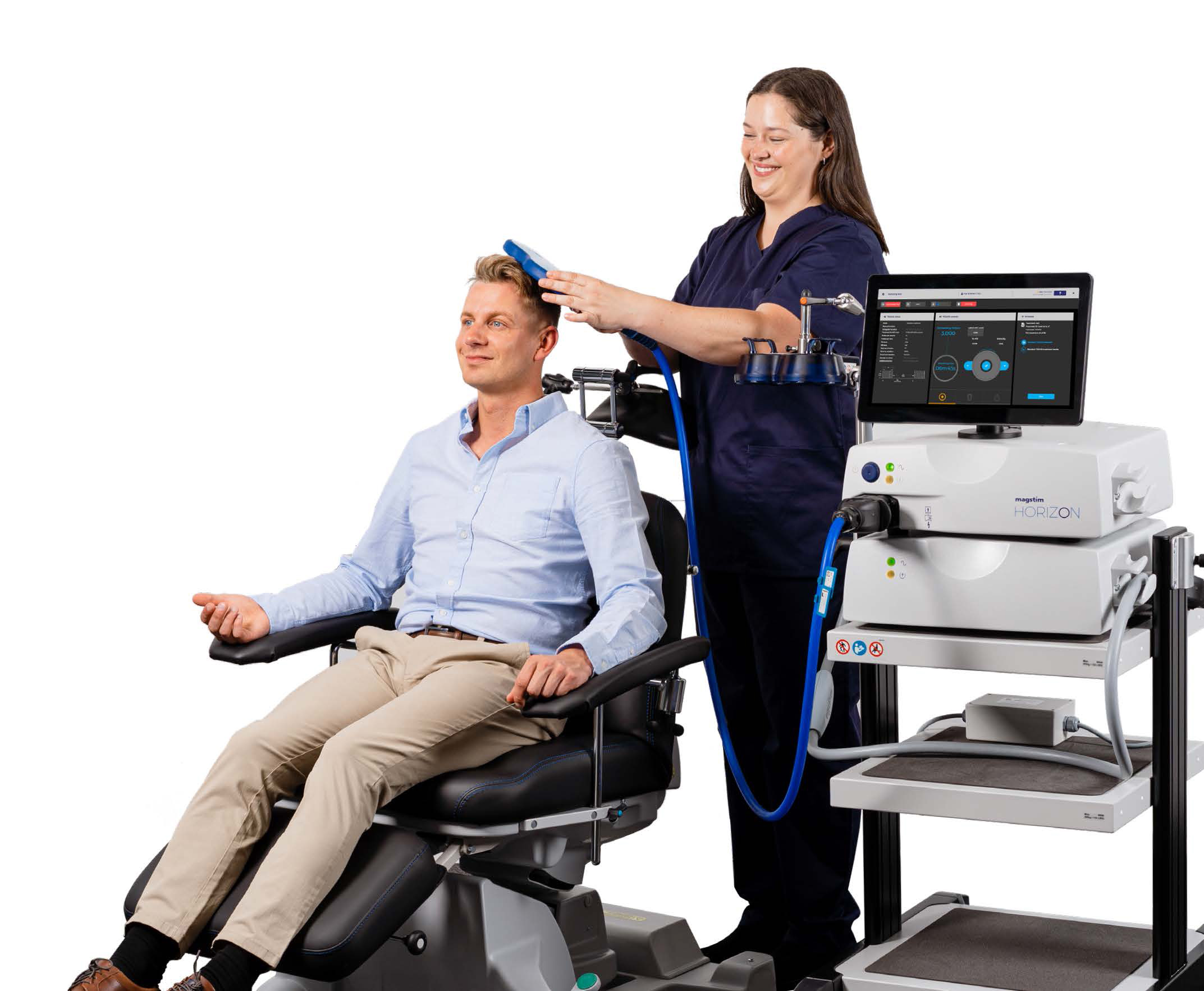
UK System for Patient Treatment of MDD, OCD and Decreasing Anxiety*
CARMARTHENSHIRE, United Kingom, Dec. 05, 2024 (GLOBE NEWSWIRE) -- Magstim, the global leader in neuroscience research and treatment for mental health, has been awarded UKCA clearance for the Horizon Inspire System, continuing to provide NHS physicians and researchers with next-generation transcranial magnetic stimulation technology to treat patients with MDD, OCD and Decreasing Anxiety*.
“As the first to introduce clinical TMS into the NHS, our team continues delivering innovative treatments to help treat depression and anxiety,” said Professor Alex O’Neill Kerr, MBChB, Medical Director of Transforming Mind Solutions. “This new system takes trusted Magstim TMS technology to the next level, improving patient outcomes.”
“Magstim has supplied the NHS with TMS technology for more than 30 years,” said Ronnie Stolec-Campo, CEO, Magstim. “Transcranial magnetic stimulation is a proven and effective treatment with minimal side effects. The Inspire enables both experienced TMS providers as well as those who are new to TMS to get up a running fast to provide patient treatments.”
The Inspire provides high-power air-cooling, ability to deliver back-to-back customizable treatments, all while being easy to use, cost-effective and offering portability between clinic rooms. Magstim TMS technology is cited in more than 20,000 peer reviewed research papers and used in hospitals, clinics and research centers worldwide.
The use of Transcranial Magnetic Stimulation for treating MDD and OCD is increasing worldwide, driven by new studies demonstrating its effectiveness over other treatments (**).
The Horizon Inspire system leverages intuitive preset clinical workflows to simplify the treatment process, delivering precise results with no pulse decay, ensuring the correct dosage. Magstim’s air-cooled coil reduces downtime and eliminates additional cooling expenses. Advanced data analytics tools improve treatment efficacy.
“Our UK based Magstim team engineered the very first commercially available TMS research technology, and we remain committed to our foundation of research” said Stolec-Campo. “We are unique in the industry because we do not charge pay per use fees, we maintain a dedicated service and support team, and we manufacture our own technology.”
Visit Magstim.com or call 844-624-7846.
About Welcony
Globally, Welcony technologies support thousands of research labs, clinics, hospitals and universities that focus on mental health, brain disorders, cognitive neuroscience and neuromonitoring. Key brands include Magstim Magnetic Stimulation, MagstimEGI high-density EEG, Technomed Clinical Neurophysiology and Neurosign Neuromonitoring. Welcony is backed by Telegraph Hill Partners, a San Francisco based private equity company.
* Horizon 3.0 Inspire is indicated for the treatment of MDD in adult patients who have failed to achieve satisfactory improvement from prior antidepressant medication in the current episode, as well as an adjunct for the treatment of adult patients suffering from Obsessive-Compulsive Disorder (OCD).
** https://www.psychiatrictimes.com/view/rtms-for-antidepressant-nonresponders
Media Contact: Mark Sejvar, mark.sejvar@magstimegi.com, +1-612-225-5868
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/a898e41a-2a63-4e21-80be-d089ca705391





