Meningococcal Vaccines Market Report 2024-2032:
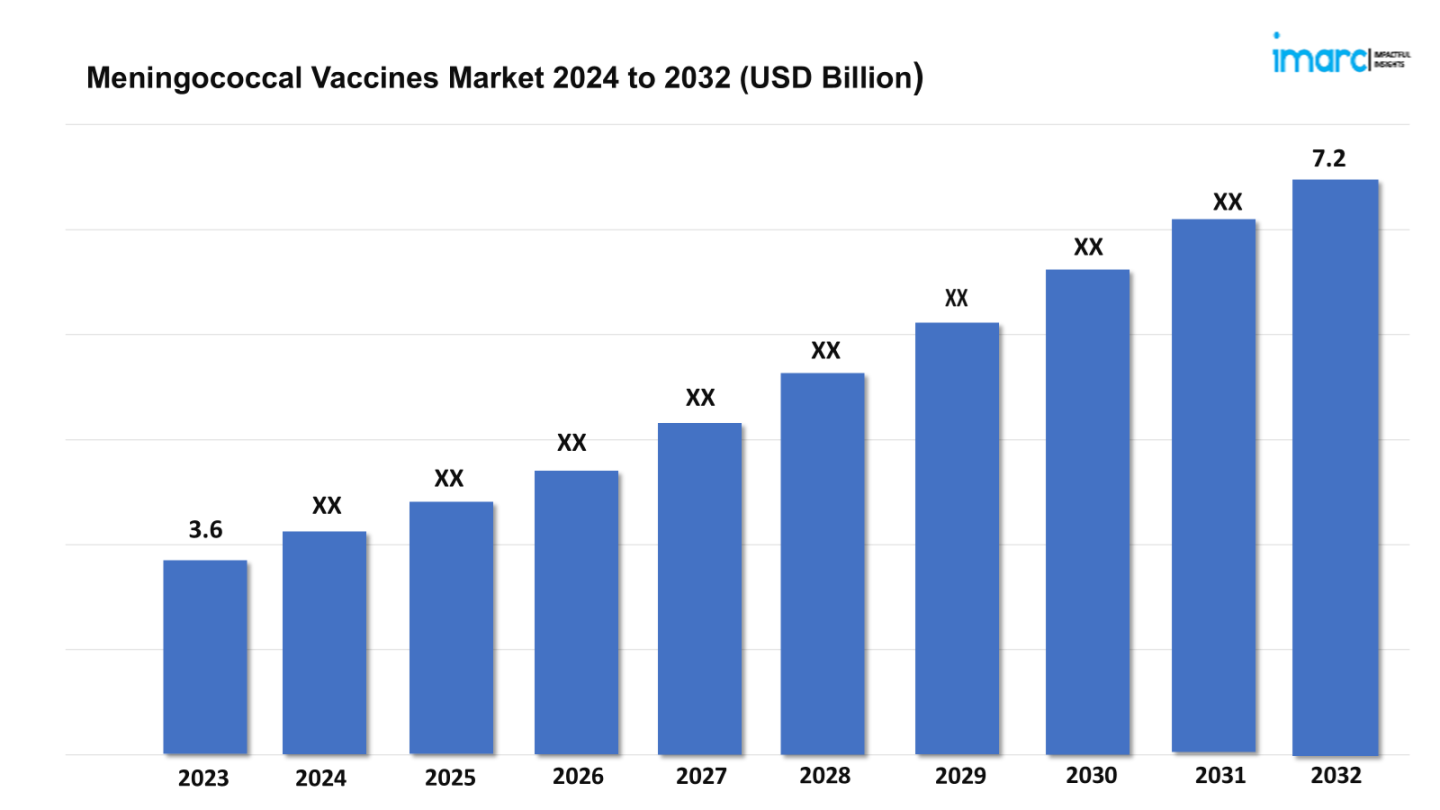
The meningococcal vaccines market size reached a value of USD 3.6 Billion in 2023. Looking forward, the market is expected to reach USD 7.2 Billion by 2032, exhibiting a growth rate (CAGR) of 7.7% during 2024-2032.
The market is driven by continual advancements in vaccine technology, favorable government initiatives and immunization programs, rising public awareness about meningococcal vaccination, growing travel and migration increasing disease spread, intensified efforts to increase vaccination coverage, development of new and improved vaccines, integration of meningococcal antigens into combination vaccines, and innovations in vaccine delivery systems.
Increasing Prevalence of Meningococcal Disease: A Key Market Driver
The global meningococcal vaccines market is significantly driven by the increasing prevalence of meningococcal disease. Meningococcal disease, caused by the bacterium Neisseria meningitidis, leads to serious conditions such as meningitis and septicemia, which can be fatal or result in severe long-term disabilities. Over the past decade, numerous outbreaks of meningococcal disease have been reported worldwide, highlighting the urgent need for effective vaccination programs. The World Health Organization (WHO) and various national health agencies have highlighted the critical importance of meningococcal vaccination, particularly in regions with high incidence rates such as sub-Saharan Africa, known as the “meningitis belt.” This area experiences frequent epidemics, especially during the dry season, resulting in high morbidity and mortality rates.
Request a PDF Sample Report: https://www.imarcgroup.com/meningococcal-vaccines-market/requestsample
The global mobility of population, including increased travel and migration, further exacerbates the spread of meningococcal disease, making vaccination a public health priority. In response to these challenges, governments and health organizations have intensified efforts to increase vaccination coverage. Immunization campaigns targeting at-risk population, such as infants, adolescents, and travelers to endemic regions, are being implemented on a large scale. Nigeria reported a 50% increase in annual meningitis cases across Africa in 2023, with a recent serogroup C outbreak resulting in 1,742 suspected cases, 101 confirmed cases, and 153 deaths, prompting the Men5CV vaccine rollout targeting over one million individuals aged 1-29, while MenB accounted for over half of IMD cases among US adolescents and young adults from 2017-2021, with less than 12% of US adolescents receiving the two required doses.
Additionally, educational initiatives aimed at raising awareness about the dangers of meningococcal disease and the benefits of vaccination are being promoted. These efforts are not only driving the demand for meningococcal vaccines but also encouraging the development of new and improved vaccines to address different serogroups of the bacterium. The rise in reported cases of meningococcal disease in both developed and developing countries is propelling research and innovation in vaccine technologies, leading to the introduction of more effective and broader-spectrum vaccines.
Continual Advancements in Vaccine Technology: Contributing to Market Growth
Continual advancements in vaccine technology are playing a pivotal role in driving the global meningococcal vaccines market. In recent years, significant strides have been made in the development of meningococcal vaccines, resulting in more effective and safer immunization options. Traditional polysaccharide vaccines, which have been used for many years, are being replaced by conjugate vaccines that offer longer-lasting immunity and are more effective in young children. Conjugate vaccines, which link the polysaccharide antigen to a protein carrier, have proven to be highly immunogenic and provide robust protection against multiple serogroups of Neisseria meningitidis. Moreover, the advent of protein-based vaccines has expanded the scope of meningococcal vaccination. These vaccines target surface proteins of the bacteria, such as factor H binding protein (fHbp), and have shown promising results in providing broad protection across different serogroups.
The use of advanced technologies such as reverse vaccinology and genomic sequencing has facilitated the identification of new vaccine targets, enabling the development of next-generation vaccines. Reverse vaccinology, for instance, involves the analysis of the entire genome of a pathogen to identify potential antigens that can induce an immune response. This innovative approach has led to the discovery of novel antigens for meningococcal vaccines, resulting in the introduction of vaccines with enhanced efficacy. Additionally, the development of combination vaccines, which protect against multiple diseases, has gained momentum. These vaccines simplify immunization schedules, increase vaccination compliance, and reduce healthcare costs. The integration of meningococcal antigens into combination vaccines, such as those including diphtheria, tetanus, and pertussis, has been particularly successful. An oropharyngeal carriage study in Buenos Aires revealed a 6.5% carriage rate among children aged 1-17, with higher rates (9.4%) in adolescents aged 10-17, associated with passive smoking and nightclub visits. Argentina implemented a routine vaccination program against N. meningitidis for infants and adolescents, with varying uptake rates: 77.4% at 3 months, decreasing to 47.5% by 11 years. Furthermore, advancements in vaccine delivery systems, such as intranasal and microneedle patch vaccines, are enhancing the accessibility and acceptability of meningococcal vaccination. These novel delivery methods offer advantages such as ease of administration, reduced pain, and improved stability, making vaccination more convenient and appealing to a broader population.
Favorable Government Initiatives and Immunization Programs: Propelling Market Expansion
Government initiatives and immunization programs are fundamental drivers of the global meningococcal vaccines market. Recognizing the significant public health burden posed by meningococcal disease, governments worldwide are implementing comprehensive strategies to enhance vaccination coverage and control outbreaks. National immunization programs are being strengthened to include meningococcal vaccines in routine vaccination schedules, ensuring that vulnerable population, such as infants and adolescents, receive timely protection against the disease. For instance, many countries have introduced meningococcal conjugate vaccines into their national immunization programs, targeting various age groups to provide broad protection. These programs are often supported by substantial government funding and international collaborations with organizations such as the World Health Organization (WHO) and the Gavi Alliance. Gavi, in particular, has been instrumental in providing financial support and technical assistance to low-income countries, enabling them to introduce and scale up meningococcal vaccination. On April 12, 2024, Nigeria became the first country to introduce the WHO-recommended 5-in-1 Men5CV vaccine against five strains of meningococcus, funded by Gavi, aiming to immunize over one million people aged 1-29 in response to a recent outbreak, marking a significant step towards eliminating meningitis in Africa by 2030.
Additionally, governments are implementing catch-up campaigns to vaccinate older children, adolescents, and young adults who may have missed routine immunization. Such campaigns are crucial in achieving herd immunity and preventing disease transmission within communities. Furthermore, governments are investing in public awareness campaigns to educate the population about the importance of meningococcal vaccination. These campaigns aim to dispel myths and misconceptions about vaccines, address vaccine hesitancy, and promote the benefits of immunization. By leveraging various communication channels, including social media, traditional media, and community outreach programs, governments are reaching diverse audiences and encouraging vaccine uptake. In response to the COVID-19 pandemic, there has been a renewed emphasis on strengthening immunization systems and ensuring the continuity of vaccination services. Governments are prioritizing the integration of meningococcal vaccination into broader public health initiatives, recognizing the critical role of vaccines in preventing disease outbreaks and safeguarding public health. Additionally, policy frameworks and regulatory support are being enhanced to facilitate the rapid approval and distribution of new meningococcal vaccines. Streamlined regulatory pathways and expedited approval processes are enabling quicker access to innovative vaccines, addressing emerging public health needs. Overall, government initiatives and immunization programs are driving the global meningococcal vaccines market by providing the necessary infrastructure, funding, and public awareness to ensure widespread vaccination coverage and control of meningococcal disease.
Buy Full Report: https://www.imarcgroup.com/checkout?id=2730&method=502
Leading Companies in the Meningococcal Vaccines Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global meningococcal vaccines market, several notable companies are continuously innovating and expanding their product portfolios. Leading firms are developing next-generation vaccines that offer broader protection and improved safety profiles. These companies are leveraging advanced technologies like reverse vaccinology and genomic sequencing to identify new vaccine targets and enhance efficacy. Additionally, they are investing in the development of combination vaccines, which simplify immunization schedules and increase compliance. Strategic collaborations and partnerships with governments and international organizations, such as the World Health Organization (WHO) and Gavi, are also a key focus, enabling wider distribution and access to vaccines in low-income regions. Public awareness campaigns and educational initiatives are being launched to promote the benefits of vaccination and address vaccine hesitancy, further driving market growth and improving public health outcomes. Some of the prominent players in the market include GlaxoSmithKline Plc, Hualan Biological Engineering Inc., Incepta Pharmaceuticals Limited, Novartis AG, Pfizer Inc., Sanofi S.A., Serum Institute of India Ltd., and Walvax Biotechnology Co. Ltd.
On 20th October, 2023, Pfizer announced that the FDA has approved PENBRAYA™, a pentavalent vaccine providing coverage against meningococcal groups A, B, C, W, and Y for adolescents and young adults aged 10 to 25. This approval is based on positive Phase 2 and Phase 3 trial results, demonstrating robust immunogenicity and a favorable safety profile.
On 16th April, 2024, GSK announced that the FDA has accepted the Biologics License Application for its 5-in-1 MenABCWY vaccine candidate, with a regulatory decision expected by February 14, 2025. This vaccine, based on positive Phase III trial results, combines components of Bexsero and Menveo to provide broad coverage against the five most common meningococcal serogroups, potentially simplifying immunization schedules.
Market Analysis:
Meningococcal vaccines play a crucial role in preventing infections caused by Neisseria meningitidis, a leading cause of bacterial meningitis and sepsis worldwide. The MenACWY vaccine targets four significant serogroups: A, C, W, and Y, providing broad protection and contributing to substantial reductions in disease incidence in populations where it’s administered. MenB vaccines, including MenB & Manic, are essential in protecting against serogroup B, a major cause of meningococcal disease outbreaks, especially in adolescents and young adults. The MenC vaccine focuses specifically on serogroup C, which has been responsible for numerous meningitis outbreaks in various regions, making it a critical component in national immunization programs.
MenA vaccine is vital in regions such as sub-Saharan Africa, where serogroup A has historically caused large epidemics. The MenAC vaccine, covering both serogroups A and C, offers a targeted approach for areas where these two serogroups are prevalent, enhancing the overall immunization strategy. Additionally, the “Others” category includes vaccines targeting less common serogroups or combination vaccines that address multiple serotypes, contributing to comprehensive protection against meningococcal disease.
These vaccines’ effectiveness in reducing morbidity and mortality drives their demand, making them pivotal in the global meningococcal vaccines market. Their broad coverage and targeted protection strategies are essential in addressing regional disease burdens, supporting public health initiatives, and ultimately driving market growth.
Request for customization: https://www.imarcgroup.com/request?type=report&id=2730&flag=E
Regional Analysis:
The major markets for meningococcal vaccines include North America (the United States and Canada); Europe (Germany, France, the United Kingdom, Italy, Spain, Russia and others); Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, and others); Latin America (Brazil, Mexico, and others); and the Middle East and Africa. According to projections by IMARC, North America represented the largest regional market.
The North American meningococcal vaccines market, encompassing the United States and Canada, is driven by high awareness of meningococcal disease and proactive vaccination programs. The region benefits from strong government initiatives, such as routine vaccination schedules for adolescents and college students, and public health campaigns promoting vaccine uptake. Additionally, the presence of leading pharmaceutical companies and advanced healthcare infrastructure supports the rapid development and distribution of vaccines.
Europe’s market includes key countries like Germany, France, the United Kingdom, Italy, Spain, and Russia. The market is characterized by comprehensive immunization programs and high public awareness. European nations often implement national vaccination strategies that include meningococcal vaccines as part of routine immunizations for children and high-risk groups.
The Asia Pacific market covers countries such as China, Japan, India, South Korea, Australia, and Indonesia. The market is witnessing rapid growth due to increasing awareness of meningococcal disease and expanding immunization programs. Government initiatives to include meningococcal vaccines in national immunization schedules, particularly in countries like China and India, are significant drivers. The rising incidence of meningococcal infections in densely populated areas necessitates effective vaccination strategies.
The Latin American market includes Brazil, Mexico, and other countries in the region. The market growth is driven by efforts to control meningococcal disease outbreaks through comprehensive vaccination programs. Government initiatives and public health campaigns play a vital role in raising awareness and promoting vaccine uptake. The region faces challenges such as limited healthcare infrastructure and varying levels of vaccine coverage, but ongoing efforts to improve immunization rates are promising.
The Middle East and Africa region face significant challenges related to meningococcal disease, particularly due to the prevalence of serogroup A. The market is driven by initiatives like the Meningitis Vaccine Project, which aims to eliminate meningitis epidemics in sub-Saharan Africa. Governments and international health organizations work together to implement large-scale vaccination campaigns, particularly targeting high-risk populations. The introduction of the MenA vaccine has significantly reduced disease incidence in the meningitis belt.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2032
Breakup by Vaccine Type:
- Conjugate
- Polysaccharide
- Subcapsular
Breakup by Composition:
- Mono Vaccines
- Combination Vaccines
Breakup by Vaccine Serotype:
- MenACWY
- MenB & Manic
- MenC
- MenA
- MenAC
- Others
Breakup by Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Institutional Sales
- Others
Breakup by End User:
- Pediatric
- Adult
Breakup by Region:
- North America
- United States
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Others
- Europe
- Germany
- France
- United Kingdom
- Italy
- Spain
- Russia
- Others
- Latin America
- Brazil
- Mexico
- Others
- Middle East and Africa
Competitive Landscape:
The market research report has provided a comprehensive analysis of the competitive landscape. Detailed profiles of all major market companies have also been provided. Some of the key players in the market include:
· GlaxoSmithKline Plc
· Hualan Biological Engineering Inc.
· Incepta Pharmaceuticals Limited
· Novartis AG
· Pfizer Inc.
· Sanofi S.A.
· Serum Institute of India Ltd.
· Walvax Biotechnology Co. Ltd.
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/meningococcal-vaccines-market
IMARC Group Offer Other Reports:
Saudi Arabia Active Pharmaceutical Ingredients (API) Market: The Saudi Arabia active pharmaceutical ingredients (API) market size is projected to exhibit a growth rate (CAGR) of 1.85% during the forecast period from 2024 to 2034.
Saudi Arabia Pharmaceutical Glass Packaging Market: The Saudi Arabia pharmaceutical glass packaging market size is projected to exhibit a growth rate (CAGR) of 4.01% during the forecast period from 2024 to 2034.
Saudi Arabia Mental Health Market: The Saudi Arabia mental health market size reached USD 210.4 Million in 2023. Looking forward, IMARC Group expects the market to reach USD 365.7 Million by 2032, exhibiting a growth rate (CAGR) of 6.12% during the forecast period from 2024 to 2034.
Saudi Arabia Health and Wellness Market: The Saudi Arabia health and wellness market size is projected to exhibit a growth rate (CAGR) of 10.08% during the forecast period from 2024 to 2034.
GCC Health and Wellness Market: The GCC health and wellness market size reached USD 67.9 Billion in 2023. Looking forward, IMARC Group expects the market to reach USD 118.6 Billion by 2032, exhibiting a growth rate (CAGR) of 6.4% during the forecast period from 2024 to 2034.
UAE Health Insurance Market: The UAE health insurance market size reached USD 8.2 Billion in 2023. Looking forward, IMARC Group expects the market to reach USD 14.6 Billion by 2032, exhibiting a growth rate (CAGR) of 6.4% during the forecast period from 2024 to 2034.
UAE Weight Loss Market: The UAE weight loss market size reached USD 1,100 Million in 2023. Looking forward, IMARC Group expects the market to reach USD 1,770 Million by 2032, exhibiting a growth rate during the forecast period from 2024 to 2034.
GCC E-Pharmacy Market: The GCC E-pharmacy market size is projected to exhibit a growth rate (CAGR) of 23.4% during the forecast period from 2024 to 2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800