Mycosis Fungoides Market Outlook 2025-2035:
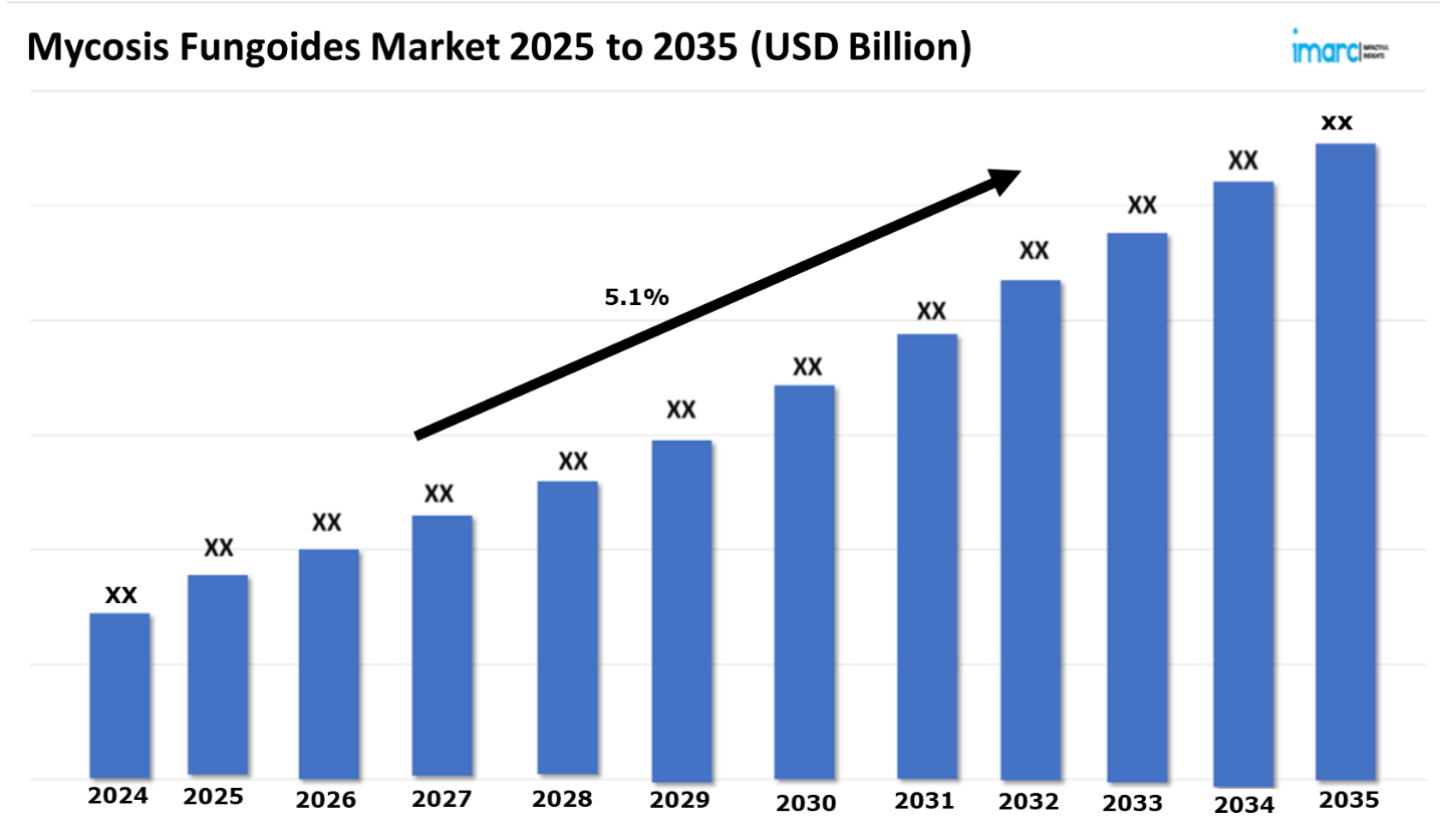
The mycosis fungoides market is demonstrating a promising trajectory, with a projected CAGR of 5.1% across the 7 major markets between 2025 and 2035. The market is driven by the escalating demand for innovative treatment modalities, including extracorporeal photopheresis, which involves the removal, treatment, and reinfusion of a patient’s blood to stimulate immune responses against cancerous cells. Moreover, advanced biologics, monoclonal antibodies, and immune checkpoint inhibitors are improving treatment outcomes by precisely targeting malignant T-cells while minimizing damage to healthy tissues. Phototherapy, particularly narrowband UVB and PUVA, remains a key non-invasive option, while novel topical therapies and targeted drug delivery systems enhance localized treatment. Additionally, gene and cellular therapies, including CAR-T cell therapy, are emerging as potential breakthroughs. These innovations are expanding treatment options, improving patient quality of life, and fueling market growth in the mycosis fungoides landscape.
Developments in Early Detection and Diagnostic Technologies: Fuelling the Market for Mycosis Fungoides Technological advancements that enable early diagnosis and detection significantly drive the mycosis fungoides market. One notable advancement in diagnosis is the usage of high-resolution skin imaging technologies. Optical coherence tomography (OCT) and reflectance confocal microscopy (RCM) non-invasively enable viewing of the skin layers and early detection and help to differentiate benign skin conditions. Moreover, dermoscopy has improved the detection of characteristic skin patterns associated with mycosis fungoides, thus aiding in early clinical suspicion. Molecular diagnostics have also transformed the diagnosis of mycosis fungoides. Clonal T-cell populations, the hallmark of CTCL, are identified through PCR-based T-cell receptor gene rearrangement studies. More recent advancements in immunohistochemistry and flow cytometry can now make an accurate diagnosis of malignant T-cell markers, increasing the degree of diagnostic specificity. Liquid biopsy is another recent tool, a minimally invasive means to identify circulating tumor DNA and monitor the disease’s progress. This approach makes possible earlier diagnosis and real-time assessment of response to treatment, which can make better disease management strategies. Advances in AI and machine learning also fuel innovation through early detection with the help of dermatological images and biopsy results, with great accuracy, leading to fewer rates of misdiagnosis. Hence, as the use of such technologies becomes increasingly prevalent, demand for advanced diagnostics and personalized approaches to treatment should grow, in turn propelling the mycosis fungoides market forward.
Request a PDF Sample Report: https://www.imarcgroup.com/mycosis-fungoides-market/requestsample
Advancement in Innovative Pharmaceutical and Therapeutic Therapies: Supporting Market Growth The market for mycosis fungoides is growing due to the creation of new therapies and pharmaceutical treatments. Traditional treatment approaches, such as corticosteroids, phototherapy, and chemotherapy, have limitations in terms of efficacy and safety, leading to increased demand for innovative therapies that provide better disease control with fewer side effects Among the most promising treatments for mycosis fungoides, targeted biologic medicines play a vital role. By increasing therapy accuracy while reducing systemic toxicity, the monoclonal antibodies targeting the receptors of cancerous T-cells in mogamulizumab enhance its precision. Other potential therapies have come through HDAC inhibitors that cause the cancerous cells to delay the course of the disease, with such options including vorinostat and romidepsin. Immunotherapy is also another huge drive for this market. Investigations have been undertaken into the applicability of vaccination for therapeutic measures and the checkpoint inhibitors of an immune system approach for boosting and promoting the actions of the latter on malignant T-cells. The potential of CAR-T cell therapy, a highly customized approach that has transformed the treatment of hematologic malignancies, is also being investigated in the context of CTCL. New drug delivery strategies, including topical medications and nanotechnology-based formulations, are also enhancing localized therapy while minimizing systemic adverse effects and increasing compliance for patients. A new combination drug for mycosis fungoides is being developed that combines immunotherapy, biologics, and traditional medicines. It promises to bring favorable results regarding enhanced global effectiveness. Given more studies and clinical trials plus regulatory support, the market size of mycosis fungoides will eventually be high in the future. Besides offering options to patients who are to get treatments, the entry of innovative drugs attracts investments in biopharmaceutical research, which will continue to progress in this field. All these developments are improving patient outcomes and driving market growth.
Buy Full Report: https://www.imarcgroup.com/checkout?id=11898&method=809
Marketed Therapies in Mycosis Fungoides Market
Adcetris (Brentuximab vedotin): Seagen/Takeda Oncology
The antibody-drug conjugate (ADC) Adcetris or Brentuximab Vedotin, is made up of a monoclonal antibody directed by CD30 attached to monomethyl auristatin E (MMAE), a chemical that disrupts microtubules, using a protease-cleavable linker and Seagen’s proprietary technology. When internalized by CD30-positive tumor cells, the linker system used by the ADC releases MMAE while remaining stable in circulation. Poteligeo (Mogamulizumab): Kyowa Kirin
A novel humanized monoclonal antibody (mAb) called POTELIGEO targets the CC chemokine receptor 4 (CCR4). Upon binding to CCR4, POTELIGEO enhances the immune system’s capacity to eradicate cancerous cells. POTELIGEO makes use of Kyowa Kirin’s unique POTELLIGENT technology, which boosts the body’s natural immunological response to therapy, resulting in increased efficacy in eliminating cancer cells.
Valchlor (Chlormethine): Actelion Pharmaceuticals/Helsinn
Valchlor (chlormethine) is a topical chemotherapeutic medication that treats mycosis fungoides. Valchlor acts as a bifunctional alkylating chemical that directly destroys the DNA of rapidly dividing malignant T cells in the skin, causing cell death (apoptosis) by producing double-stranded DNA breaks and suppressing DNA repair pathways. It successfully targets malignant T cells that accumulate in patients’ epidermis with mycosis fungoides.
Emerging Therapies in Mycosis Fungoides Market
AFM13: Affimed Therapeutics
CD16A on NK cells and macrophages and CD30 on tumor cells are the binding sites for the new tetravalent, bispecific innate cell engager AFM13. The role of AFM13 in peripheral T-cell lymphoma and other CD30-positive lymphomas is being studied. Through the engagement and activation of NK cells and macrophages, AFM13 leverages the innate immune system to cause the targeted and selective death of CD30-positive tumor cells. When used as a monotherapy for CD30-positive non-Hodgkin lymphoma with cutaneous symptoms, AFM13 has a great safety record and indications of therapeutic impact. HyBryte: Soligenix
HyBryte is a photodynamic therapy that combines synthetically generated hypericin in an ointment with visible fluorescent light. Synthetic hypericin, the active ingredient in HyBryte, accumulates in T-cells. Once hypericin is present in T-cells, it can be triggered by safe, visible fluorescent light. When synthetic hypericin is activated, it produces oxygen radicals, which cause cellular toxicity and kill the targeted T-cells.
Drug Name | Company Name | MOA | ROA |
AFM13 | Affimed Therapeutics | Antibody-dependent cell cytotoxicity; Natural killer cell stimulants | Intravenous |
HyBryte | Soligenix | Cell death stimulants; HSP90 heat-shock protein inhibitors; Photosensitisers; Protein kinase C inhibitors; Singlet oxygen stimulants | Topical |
Detailed list of emerging therapies in Mycosis Fungoides is provided in the final report…
Leading Companies in the Mycosis Fungoides Market:
The competitive environment in the industry is thoroughly examined in the IMARC market research study. Several industry leaders are at the forefront of creating integrated platforms to improve mycosis fungoides management in the worldwide market. Kyowa Kirin, Takeda Oncology, and Seagen are a few of the prominent participants. Through ongoing research, the development of diagnostic tools, and the expansion of their product lines to satisfy the rising demand for the disease, these businesses are propelling innovation in the mycosis fungoides market.
In January 2025, the reimbursement of POTELIGEO (mogamulizumab) for adult patients with mycosis fungoides has been approved by the Bulgarian Ministry of Health and the Croatian National Health Insurance Fund (NHIF), according to an announcement from Kyowa Kirin International and Swixx BioPharma AG.
In November 2023, Seagen Inc. announced that progression-free survival findings for a combination of immunotherapy and ADCETRIS (brentuximab vedotin) in early and advanced-stage classical Hodgkin lymphoma (cHL) will be reported at 12 and 24 months, respectively.
Request for customization: https://www.imarcgroup.com/request?type=report&id=11898&flag=E
Key Players in Mycosis Fungoides Market:
The key players in the Mycosis Fungoides market who are in different phases of developing different therapies are Seagen, Takeda Oncology, Kyowa Kirin, Actelion Pharmaceuticals, Helsinn, Therakind, Affimed Therapeutics, Soligenix, 4SC, Innate Pharma, Myeloid Therapeutics, and Others.
Regional Analysis:
The most prominent markets for mycosis fungoides are the US, Germany, France, the UK, Italy, Spain, and Japan. According to the IMARC forecast, the United States is the biggest market for mycosis fungoides therapy and houses the biggest patient population suffering from the condition. This is in all probability due to the rising application of advanced and innovative therapeutic methods that have improved patient results, like immunotherapy, histone deacetylase inhibitors, and monoclonal antibodies.
The aging population and the incidence of immune-related diseases further spur demand for mycosis fungoides. Due to the improvements in healthcare infrastructures, increased availability of treatments, and enhanced insurance coverages in the United States, patients can now easily access highly advanced medicines.
The U.S. market is also driven by other factors, such as strong research funding and efforts on the part of top pharmaceutical firms and academic institutions toward clinical trials. The support from the FDA for orphan drug designations and fast-track approvals, together with the presence of large biotech companies investing in drug discovery and new medicines, accelerates the entry of state-of-the-art therapies.
Recent Developments in Mycosis Fungoides Market:
· In January 2025: An intermediate update on the open-label, investigator-initiated trial (IIS) examining prolonged HyBryte (synthetic hypericin) therapy for up to 12 months in patients with early-stage cutaneous T-cell lymphoma was provided by Soligenix, Inc.
· In June 2024: Innate Pharma SA released encouraging results from the Phase 2 TELLOMAK trial of lacutamab in mycosis fungoides. Regardless of the initial level of KIR3DL2 expression, lacutamab therapy significantly reduces tumor activity in patients with mycosis fungoides who have had intensive pretreatment. Lacutamab had a safety profile similar to earlier research and was well tolerated.
Key information covered in the report.
Base Year: 2024
Historical Period: 2019-2024
Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the mycosis fungoides market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the mycosis fungoides market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current mycosis fungoides marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC: https://www.imarcgroup.com/mycosis-fungoides-market/toc
IMARC Group Offer Other Reports:
Non Hodgkin’s Lymphoma Market: The 7 major Non Hodgkin’s lymphoma market reached a value of US$ 4.0 Billion in 2023, and projected the 7MM to reach US$ 7.4 Billion by 2034, exhibiting a growth rate (CAGR) of 5.71% during 2024-2034.
B-Cell Non-Hodgkin Lymphoma Market: The 7 major B-cell non-Hodgkin lymphoma market are expected to exhibit a CAGR of 5.83% during 2024-2034.
T-Cell Non-Hodgkin Lymphoma Market: The 7 major T-cell non-Hodgkin lymphoma market are expected to exhibit a CAGR of 5.55% during 2024-2034.
Inflammatory Bowel Disease Market: The 7 major inflammatory bowel disease market reached a value of USD 14.92 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 20.08 Billion by 2035, exhibiting a growth rate (CAGR) of 2.73% during 2025-2035.
Hodgkin’s Lymphoma Market: The 7 major Hodgkin’s lymphoma market reached a value of US$ 6.4 Billion in 2023, and projected the 7MM to reach US$ 18.9 Billion by 2034, exhibiting a growth rate (CAGR) of 10.29% during 2024-2034.
Marginal Zone Lymphoma Market: The 7 major marginal zone lymphoma market reached a value of US$ 1.7 Billion in 2023, and projected the 7MM to reach US$ 2.7 Billion by 2034, exhibiting a growth rate (CAGR) of 4.61% during 2024-2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800