Study offers promise of greater comfort for current CPAP users and a better option for the roughly half of obstructive sleep apnea patients who refuse or abandon CPAP
MURFREESBORO, Tenn., Oct. 8, 2024 /PRNewswire/ -- A clinical study of the substantially lower pressure applied by a new CPAP device for treating obstructive sleep apnea (OSA) has found it to be just as effective as the continuous pressure of traditional CPAP while also finding most users report it as more comfortable, holding out hope for millions of OSA patients who find CPAP intolerable and quit using it.
Results of the study comparing the new technology—known as KPAP™ and developed by SleepRes™—to traditional continuous positive airway pressure (CPAP) have been published online by the journal Sleep Medicine.
Nearly 50 million Americans live with OSA, putting them at increased risk for heart attack, stroke, high blood pressure, heart failure, and falling asleep while driving. CPAP is widely used by healthcare professionals to treat the condition, and the technology has proven successful in reducing OSA events, which obstruct a patient's airway during sleep.
But around half of patients who begin traditional CPAP discontinue its use because of problems they experience with it. The biggest of these are the breathing discomfort associated with the constant airway pressure and air leak around the mask.
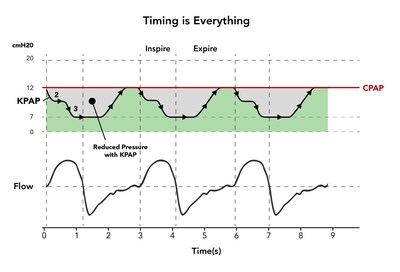
In this study, pressure applied during the breathing cycle by KPAP was reduced by up to 5 cm of water (H2O) below the level a patient would normally receive from traditional CPAP. In doing so, it was found that KPAP reduced the leak by half. For example, a patient normally requiring 10 cm H2O with traditional CPAP would experience only 5 cm H2O during most of the respiratory cycle with KPAP. While not part of the study, researchers expect to ultimately find that reduced pressure and leak will lead to more patients being able to better tolerate the therapy.
"CPAP has been considered the gold-standard of care for OSA for nearly 40 years, yet over that time, all attempts to improve comfort with CPAP have involved reducing pressure during exhalation, which has not led to improved patient adherence to therapy. This study showed that lowering pressure during inhalation, which can make breathing feel more natural, does not affect the therapy's effectiveness and is overwhelmingly reported to be more comfortable," said David P. White, MD, professor of medicine at Harvard Medical School and one of the authors of the study. "We're excited about this initial study and about the potential this technology has for helping many people who currently find CPAP intolerable or just uncomfortable."
Different from CPAP, which provides consistent, full pressure throughout the breathing cycle, the KPAP algorithm applies multiple drops in pressure during inhalation, also known as inspiration, only to return to full pressure at the "right time," near the end of exhalation. The new technology is called Kairos PAP or KPAP, coming from the Greek word Kairos, meaning "at the right time," thus referring to when pressure is applied during a sleeping patient's breathing cycle.
The study included two randomized clinical trials among participants aged 18 to 70 with a recent diagnosis (within 1 year) of OSA. The Efficacy trial compared the effect of KPAP and CPAP on the Apnea-Hypopnea Index (AHI), the primary diagnostic tool for measuring the severity of OSA, expressed in the number of OSA events (apneas and hypopneas) a patient experiences per hour of sleep. In the second trial, known as the Comfort trial, a range of differing pressure levels was applied to test the preference of awake patients for KPAP versus CPAP.
In the Efficacy trial, 48 patients with CPAP experience were studied in a lab using a standard polysomnogram and a split-night design, meaning 3.5 hours each on KPAP and CPAP, the order being randomly assigned. KPAP was found to reduce AHI more than CPAP by a mean difference of 0.5 events per hour (P=0.007), a statistically significant reduction that—while not clinically meaningful—confirmed that KPAP provides efficacy comparable to CPAP. Unintentional leak, which is CPAP air leakage occurring around the mask or through the mouth, was also reduced by KPAP by more than 50 percent (P<0.001) compared to CPAP.
In the Comfort trial, 150 patients with no CPAP experience were assessed in-office on their preference for KPAP versus CPAP. The trial's two primary endpoints were comparisons of patients whose preference for KPAP or CPAP was assessed on two different baseline pressures of 9 and 13 cm H20.
During the tests, these two baseline pressure levels remained continuous for CPAP throughout the participants' breathing cycles. But with KPAP, the pressure at the 9 cm H2O level was dropped by a total of 4 cm during inspiration while the pressure at the 13 cm H2O level was dropped by a total of 5 cm during inspiration. Researchers found that at the 9 cm and 13 cm baseline levels, 69 percent and 84 percent, respectively, of participants preferred KPAP over CPAP (P<0.001).
In addition to these endpoints, researchers also measured a range of other KPAP pressure drops from both the 9 cm and 13 cm baselines. Overall, when data was combined for all levels, 93 percent of patients preferred KPAP over CPAP for the 9 cm H2O baseline while 95 percent preferred KPAP for the 13 cm H20 baseline.
"As a sleep medicine physician, I am most excited about providing KPAP to my own patients," said William Noah, MD, inventor of KPAP and Chief Science Officer for SleepRes. "Because CPAP is initially difficult for most patients to tolerate and 93 to 95 percent of new patients prefer KPAP, which provides the same therapy with only half the leak, we anticipate many physicians will ultimately want to ensure that the CPAP devices they prescribe for their patients will have KPAP as an option."
To access the article in Sleep Medicine, visit https://authors.elsevier.com/c/1jqw-4y2Nr8kYA.
To learn more about KPAP, visit www.SleepRes.com.
*Currently, KPAP™ is for investigational use only and is not for sale in the U.S.
About SleepRes, Inc.
SleepRes, based in Murfreesboro, Tennessee, is developing game-changing technologies aimed at improving outcomes for patients with pulmonary and sleep disorders. The company has developed KPAP™, a first-of-its-kind algorithm aimed at reducing pressure for comfort, and still providing therapy at precisely the right time. For more information, visit the SleepRes website and follow the company on LinkedIn.
Media Contact:
Jeff Christensen
SignalWest Public Relations
(831) 566-0275
Jeff@SignalWestPR.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/new-cpap-device-with-much-lower-pressure-found-as-effective-as-traditional-cpap-and-reported-to-be-more-comfortable-302269437.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/new-cpap-device-with-much-lower-pressure-found-as-effective-as-traditional-cpap-and-reported-to-be-more-comfortable-302269437.html
SOURCE SleepRes