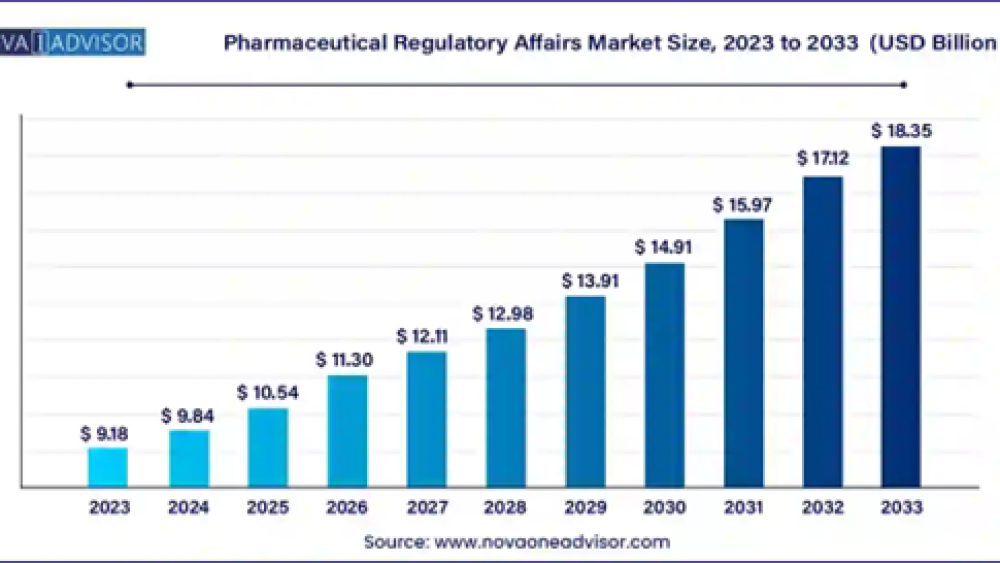
According to Nova One Advisor, the global pharmaceutical regulatory affairs market size was exhibited at USD 9.18 billion in 2023 and is projected to hit around USD 18.35 billion by 2033, growing at a CAGR of 7.17% during the forecast period 2024 to 2033.
The pharmaceutical regulatory affairs market represents collected information about a market within a various industry. The market included analysis in terms of both qualitative and quantitative data. The growth of the markets is driven by rising demand for pharmaceutical regulatory affairs leading to outsourcing and in-house applications across the globe. The increasingly changing regulatory landscape is expected to drive the market growth.
Full Report is Ready | Ask here for Sample Copy@ https://www.novaoneadvisor.com/report/sample/8795
Pharmaceutical Regulatory Affairs Market at a Glance
The increasing demand for regulatory consulting services in the pharmaceutical industry, the increasing importance of legal representation in regulatory affairs, the increasing demand for regulatory specialization in pharmaceutical fields, and increasing new product development, acquisitions, and collaborations among various companies are expected to drive the market growth.
In addition, the growing number of clinical trial registrations, increasing prevalence of existing diseases such as neurological diseases, CVDs, and cancer, increasing geriatric population across the globe, rising technological advancements in the pharmaceutical industry, and increasing government strict regulations on quality and safety standards are further anticipated to enhance the growth of the pharmaceutical regulatory affairs market during the forecast period.
Pharmaceutical Regulatory Affairs Market Key Takeaways
Increasing demand for regulatory consulting services to fuel the market growth
In the pharmaceutical industry, the increasing demand for regulatory consulting services has contributed to significant growth, enhanced by the need for compliance and the complexities of regulatory frameworks. Market analysis and industry reports offer proof of continuous growth in the demand for consulting services and represent the pharmaceutical industry’s presence of expert guidance to improve regulatory requirements. In addition, the pharmaceutical industry has also contributed to the expansion of the requirements for clinical trial applications and product registration. Evidence from industry trends and regulatory updates underscores the increasing documentation and scrutiny needed for clinical trials and product approvals. These factors are expected to drive the growth of the pharmaceutical regulatory affairs market.
However, challenges in regulatory publishing and writing may restrain market growth.
Major challenges in regulatory writing and publishing have emerged as a significant restraint, despite the overall growth in regulatory affairs services. Evidence from expert analysis and industry insights represent that the intricate nature of regulatory documentation creates obstacles for service providers and companies. These factors are responsible for obstructing the need for innovative solutions to meet complexities related to regulatory writing and publishing. Thus, these factors are expected to restrain the growth of the pharmaceutical regulatory affairs market.
Artificial intelligence in pharmaceutical market to boost the market’s growth
Artificial intelligence deals with the capability of a computer to carry out tasks related to human intelligence, such as learning, discovering, and thinking from prior experience. Artificial intelligence can be implemented to simplify the complications of pharmaceutical regulatory affairs. AI identifies vulnerabilities and reduces the time spent on quality management. In regulatory compliance, human-AI interaction opens up new opportunities. In pharmaceutical regulatory affairs, the potential role of AI is explored. AI tools can be applied to automate regulatory procedures, such as quality management, the implementation of regulations, data extraction auditing, dossier filling, and administrative work. AI reduces complexity and creates process links. These factors are expected to enhance the growth of the pharmaceutical regulatory affairs market in the coming years.
Buy Now Full Report: https://www.novaoneadvisor.com/report/checkout/8795
By service provider type, the outsourcing segment led the market
The outsourcing segment is dominated the market share in 2023 and is also expected to witness the fastest growth during the forecast period. The segment growth is attributed to the rising popularity of these services as outsourcing allows pharmaceutical companies to provide greater flexibility, improve overall efficiency, reduce staff training time, prioritize strategic projects, and reduce costs.
By category type, the drug segment led the market.
The drugs segment held the largest market share in 2023. The major Factors contributing to segment are considering to segment growth are the rising complications of drug regulations, especially for high-risk and novel therapies to ensure accelerated compliance approval. These factors are expected to drive the segment growth in the market.
North America dominated the pharmaceutical regulatory affairs market in 2023
The market is driven by the growing stringency and complexity of regulations from agencies such as the FDA that demand specialized expertise to comply and navigate with these standards. The robust biotechnology and pharmaceutical sectors in the region, marked by numerous drug development projects and research and development investments, further drive the demand for comprehensive regulatory support. The U.S. and Canada are the major countries in the region. There is a strong demand for cost-effective biosimilar and generic products in the U.S., along with patented and branded products. There has been a potential growth in the import of biosimilar and generic products from developing countries, thereby raising the demand for regulatory service suppliers in the U.S. These factors are expected to drive the growth of the pharmaceutical regulatory affairs market in North America.
Asia Pacific is expected to grow at a significant rate during the forecast period
The increasing availability of a skilled workforce at a lower cost, an increasing number of biopharmaceutical companies in the region, a growing number of clinical trials conducted in the region, and improved cost savings and regulatory landscape are expected to drive the growth of the pharmaceutical regulatory affairs market in the region. China, India, Japan, and South Korea are the major countries in the market. China is the fastest-growing country in the world. The increasingly large pool of middle-income group population and the increasing geriatric population are increasing the demand for cost-effective and innovative medicines, which is anticipated to attract major medical and biopharmaceutical device companies in China.
Immediate Delivery Available, Get Full Access@ https://www.novaoneadvisor.com/report/checkout/8795
Pharmaceutical Regulatory Affairs Market Top Key Companies:
The following are the leading companies in the pharmaceutical regulatory affairs market. These companies collectively hold the largest market share and dictate industry trends.
· In August 2024, a nonprofit advocate for nationwide health IT interoperability, the Sequoia Project launched a pharmacy workgroup as part of its Interoperability Matters program. The aim behind this launch was to identify solutions for pharmacy interoperability gaps to drive patient-centered care.
· In May 2024, a leading regulatory information management software for the medtech industry, Rimsys announced the beta launch of its community-driven, centralized hub for global regulatory intelligence data, Rimsys Intel.
· In November 2023, the AI operating system was launched by BioPhy. BioPhy’s AI platform is designed to assess biological feasibility and predict the likelihood a clinical trial will have a positive outcome, steering capital allocation and expediting time to market, by combining scientific, clinical, and regulatory insights with a proprietary operational assessment model.
Pharmaceutical Regulatory Affairs Market Report Segmentation
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Nova one advisor, Inc. has segmented the Pharmaceutical Regulatory Affairs market.
By Service Provider
https://www.novaoneadvisor.com/report/checkout/8795
Related report
Clinical Trials Market: The global clinical trials market size was estimated at USD 81.90 billion in 2023 and is projected to hit around USD 153.59 billion by 2033, growing at a CAGR of 6.49% during the forecast period from 2024 to 2033.
Biotechnology Market : The global biotechnology market size was estimated at USD 1.54 Trillion in 2023 and is projected to hit around USD 5.68 Trillion by 2033, growing at a CAGR of 13.95% during the forecast period from 2024 to 2033.
Clinical Trial Supplies Market: The clinical trial supplies market size was exhibited at USD 3.55 billion in 2023 and is projected to hit around USD 8.49 billion by 2033, growing at a CAGR of 9.11% during the forecast period 2024 to 2033.
Biosimilars Market: The global biosimilars market size was valued at USD 29.45 billion in 2023 and is anticipated to reach around USD 150.26 billion by 2033, growing at a CAGR of 17.7% from 2024 to 2033.
Biologics Market: The global biologics market size was estimated at USD 511.04 billion in 2023 and is projected to hit around USD 1,374.51 billion by 2033, growing at a CAGR of 10.4% during the forecast period from 2024 to 2033.
Oncology Market: The global oncology market size was estimated at USD 222.36 billion in 2023 and is projected to hit around USD 521.60 billion by 2033, growing at a CAGR of 8.9% during the forecast period from 2024 to 2033.
Pharma 4.0 Market: The global pharma 4.0 market size was valued at USD 14.95 billion in 2023 and is anticipated to reach around USD 79.58 billion by 2033, growing at a CAGR of 18.2% from 2024 to 2033.
U.S. Pharmaceutical Market: The U.S. pharmaceutical market size was valued at USD 602.19 billion in 2023 and is projected to surpass around USD 1,093.79 billion by 2033, registering a CAGR of 6.15% over the forecast period of 2024 to 2033.
Call: USA: +1 650 460 3308 | IND: +91 87933 22019 |Europe: +44 2080772818
Email: sales@novaoneadvisor.com
Web: https://www.novaoneadvisor.com/
The pharmaceutical regulatory affairs market represents collected information about a market within a various industry. The market included analysis in terms of both qualitative and quantitative data. The growth of the markets is driven by rising demand for pharmaceutical regulatory affairs leading to outsourcing and in-house applications across the globe. The increasingly changing regulatory landscape is expected to drive the market growth.
Full Report is Ready | Ask here for Sample Copy@ https://www.novaoneadvisor.com/report/sample/8795
Pharmaceutical Regulatory Affairs Market at a Glance
The increasing demand for regulatory consulting services in the pharmaceutical industry, the increasing importance of legal representation in regulatory affairs, the increasing demand for regulatory specialization in pharmaceutical fields, and increasing new product development, acquisitions, and collaborations among various companies are expected to drive the market growth.
In addition, the growing number of clinical trial registrations, increasing prevalence of existing diseases such as neurological diseases, CVDs, and cancer, increasing geriatric population across the globe, rising technological advancements in the pharmaceutical industry, and increasing government strict regulations on quality and safety standards are further anticipated to enhance the growth of the pharmaceutical regulatory affairs market during the forecast period.
Pharmaceutical Regulatory Affairs Market Key Takeaways
- The North America pharmaceutical regulatory affairs market held a significant global revenue share of 40.94% in 2023.
- Asia Pacific pharmaceutical regulatory affairs market is expected to grow at the fastest CAGR over the forecast period.
- The outsourcing segment dominated the market with the largest revenue share of 58.86% in 2023.
- The in-house segment is expected to grow at a considerable CAGR over the forecast period.
- The regulatory writing & publishing segment dominated the market in 2023.
- The legal representation segment is anticipated to grow at the fastest CAGR over the anticipated time period.
- The drugs segment held the largest market share in 2023.
- The biologics segment is anticipated to witness lucrative growth potential over the forecast period.
- The oncology segment dominated the market in 2023.
- The immunology segment is anticipated to project highest growth potential over the estimated timeframe
- The clinical studies segment held the largest market share in 2023.
- The preclinical segment is anticipated to grow at the fastest CAGR over the forecast period.
- The medium size companies segment dominated the market in 2023.
Increasing demand for regulatory consulting services to fuel the market growth
In the pharmaceutical industry, the increasing demand for regulatory consulting services has contributed to significant growth, enhanced by the need for compliance and the complexities of regulatory frameworks. Market analysis and industry reports offer proof of continuous growth in the demand for consulting services and represent the pharmaceutical industry’s presence of expert guidance to improve regulatory requirements. In addition, the pharmaceutical industry has also contributed to the expansion of the requirements for clinical trial applications and product registration. Evidence from industry trends and regulatory updates underscores the increasing documentation and scrutiny needed for clinical trials and product approvals. These factors are expected to drive the growth of the pharmaceutical regulatory affairs market.
However, challenges in regulatory publishing and writing may restrain market growth.
Major challenges in regulatory writing and publishing have emerged as a significant restraint, despite the overall growth in regulatory affairs services. Evidence from expert analysis and industry insights represent that the intricate nature of regulatory documentation creates obstacles for service providers and companies. These factors are responsible for obstructing the need for innovative solutions to meet complexities related to regulatory writing and publishing. Thus, these factors are expected to restrain the growth of the pharmaceutical regulatory affairs market.
Artificial intelligence in pharmaceutical market to boost the market’s growth
Artificial intelligence deals with the capability of a computer to carry out tasks related to human intelligence, such as learning, discovering, and thinking from prior experience. Artificial intelligence can be implemented to simplify the complications of pharmaceutical regulatory affairs. AI identifies vulnerabilities and reduces the time spent on quality management. In regulatory compliance, human-AI interaction opens up new opportunities. In pharmaceutical regulatory affairs, the potential role of AI is explored. AI tools can be applied to automate regulatory procedures, such as quality management, the implementation of regulations, data extraction auditing, dossier filling, and administrative work. AI reduces complexity and creates process links. These factors are expected to enhance the growth of the pharmaceutical regulatory affairs market in the coming years.
Buy Now Full Report: https://www.novaoneadvisor.com/report/checkout/8795
By service provider type, the outsourcing segment led the market
The outsourcing segment is dominated the market share in 2023 and is also expected to witness the fastest growth during the forecast period. The segment growth is attributed to the rising popularity of these services as outsourcing allows pharmaceutical companies to provide greater flexibility, improve overall efficiency, reduce staff training time, prioritize strategic projects, and reduce costs.
By category type, the drug segment led the market.
The drugs segment held the largest market share in 2023. The major Factors contributing to segment are considering to segment growth are the rising complications of drug regulations, especially for high-risk and novel therapies to ensure accelerated compliance approval. These factors are expected to drive the segment growth in the market.
North America dominated the pharmaceutical regulatory affairs market in 2023
The market is driven by the growing stringency and complexity of regulations from agencies such as the FDA that demand specialized expertise to comply and navigate with these standards. The robust biotechnology and pharmaceutical sectors in the region, marked by numerous drug development projects and research and development investments, further drive the demand for comprehensive regulatory support. The U.S. and Canada are the major countries in the region. There is a strong demand for cost-effective biosimilar and generic products in the U.S., along with patented and branded products. There has been a potential growth in the import of biosimilar and generic products from developing countries, thereby raising the demand for regulatory service suppliers in the U.S. These factors are expected to drive the growth of the pharmaceutical regulatory affairs market in North America.
Asia Pacific is expected to grow at a significant rate during the forecast period
The increasing availability of a skilled workforce at a lower cost, an increasing number of biopharmaceutical companies in the region, a growing number of clinical trials conducted in the region, and improved cost savings and regulatory landscape are expected to drive the growth of the pharmaceutical regulatory affairs market in the region. China, India, Japan, and South Korea are the major countries in the market. China is the fastest-growing country in the world. The increasingly large pool of middle-income group population and the increasing geriatric population are increasing the demand for cost-effective and innovative medicines, which is anticipated to attract major medical and biopharmaceutical device companies in China.
Immediate Delivery Available, Get Full Access@ https://www.novaoneadvisor.com/report/checkout/8795
Pharmaceutical Regulatory Affairs Market Top Key Companies:
The following are the leading companies in the pharmaceutical regulatory affairs market. These companies collectively hold the largest market share and dictate industry trends.
- Freyr
- IQVIA Inc
- ICON plc
- WuXi AppTec (WAI)
- Charles River Laboratories International, Inc.
- Labcorp Drug Development
- Parexel International Corporation
- Pharmalex GmbH
- Pharmexon
- Genpact
· In August 2024, a nonprofit advocate for nationwide health IT interoperability, the Sequoia Project launched a pharmacy workgroup as part of its Interoperability Matters program. The aim behind this launch was to identify solutions for pharmacy interoperability gaps to drive patient-centered care.
· In May 2024, a leading regulatory information management software for the medtech industry, Rimsys announced the beta launch of its community-driven, centralized hub for global regulatory intelligence data, Rimsys Intel.
· In November 2023, the AI operating system was launched by BioPhy. BioPhy’s AI platform is designed to assess biological feasibility and predict the likelihood a clinical trial will have a positive outcome, steering capital allocation and expediting time to market, by combining scientific, clinical, and regulatory insights with a proprietary operational assessment model.
Pharmaceutical Regulatory Affairs Market Report Segmentation
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, Nova one advisor, Inc. has segmented the Pharmaceutical Regulatory Affairs market.
By Service Provider
- In-house
- Outsourcing
- Regulatory Consulting
- Legal Representation
- Regulatory Writing & Publishing
- Writing
- Publishing
- Product Registration & Clinical Trial Applications
- Other Services
- Drugs
- Innovator
- Preclinical
- Clinical
- Post Market Approval (PMA)
- Generics
- Preclinical
- Clinical
- Post Market Approval (PMA)
- Innovator
- Biologics
- Biotech
- Preclinical
- Clinical
- Post Market Approval (PMA)
- ATMP
- Preclinical
- Clinical
- Post Market Approval (PMA)
- Biosimilars
- Preclinical
- Clinical
- Post Market Approval (PMA)
- Biotech
- Oncology
- Neurology
- Cardiology
- Immunology
- Others
- Preclinical
- Clinical
- Post Market Approval (PMA)
- Small
- Medium
- Large
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)
https://www.novaoneadvisor.com/report/checkout/8795
Related report
Clinical Trials Market: The global clinical trials market size was estimated at USD 81.90 billion in 2023 and is projected to hit around USD 153.59 billion by 2033, growing at a CAGR of 6.49% during the forecast period from 2024 to 2033.
Biotechnology Market : The global biotechnology market size was estimated at USD 1.54 Trillion in 2023 and is projected to hit around USD 5.68 Trillion by 2033, growing at a CAGR of 13.95% during the forecast period from 2024 to 2033.
Clinical Trial Supplies Market: The clinical trial supplies market size was exhibited at USD 3.55 billion in 2023 and is projected to hit around USD 8.49 billion by 2033, growing at a CAGR of 9.11% during the forecast period 2024 to 2033.
Biosimilars Market: The global biosimilars market size was valued at USD 29.45 billion in 2023 and is anticipated to reach around USD 150.26 billion by 2033, growing at a CAGR of 17.7% from 2024 to 2033.
Biologics Market: The global biologics market size was estimated at USD 511.04 billion in 2023 and is projected to hit around USD 1,374.51 billion by 2033, growing at a CAGR of 10.4% during the forecast period from 2024 to 2033.
Oncology Market: The global oncology market size was estimated at USD 222.36 billion in 2023 and is projected to hit around USD 521.60 billion by 2033, growing at a CAGR of 8.9% during the forecast period from 2024 to 2033.
Pharma 4.0 Market: The global pharma 4.0 market size was valued at USD 14.95 billion in 2023 and is anticipated to reach around USD 79.58 billion by 2033, growing at a CAGR of 18.2% from 2024 to 2033.
U.S. Pharmaceutical Market: The U.S. pharmaceutical market size was valued at USD 602.19 billion in 2023 and is projected to surpass around USD 1,093.79 billion by 2033, registering a CAGR of 6.15% over the forecast period of 2024 to 2033.
Call: USA: +1 650 460 3308 | IND: +91 87933 22019 |Europe: +44 2080772818
Email: sales@novaoneadvisor.com
Web: https://www.novaoneadvisor.com/