- PharmaJet needle-free technology can improve DNA vaccine delivery1 and has been successfully adopted into multiple cancer vaccine development programs.
- Partner study data shows how PharmaJet technology can outperform needle and syringe while providing safety, efficacy, and improved patient comfort.
- PharmaJet continues to execute on its strategy to be the best-in-class delivery system for novel DNA and RNA cancer immunotherapies.
GOLDEN, Colo.--(BUSINESS WIRE)--PharmaJet®, a company that strives to improve the performance and outcomes of injectables with its enabling needle-free technology, today announced their upcoming poster presentation on November 8, 2024 at the Society of Immunotherapy of Cancer (SITC) conference. The poster (#741), entitled Modulating Immune Responses to Therapeutic Cancer Vaccines through Precision Delivery Technologies, will be presented by Gregg Wilson, PhD, RN, Director of Medical and Scientific Affairs, PharmaJet. The SITC conference will be held at the George R. Brown Convention Center in Houston, Texas, bringing together leading cancer immunotherapy researchers, clinicians, scientists and industry leaders in the oncology field.
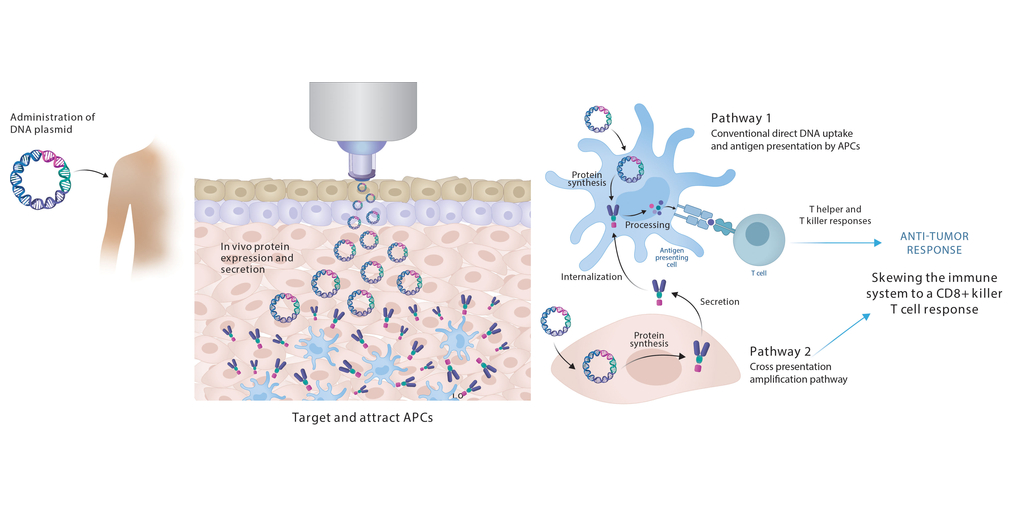


Immunotherapeutic strategies for cancer treatment include nucleic acid platforms with antigen presenting cell (APC)-targeting to boost T cell activation and the addition of neo-antigenic epitopes. Combined with checkpoint inhibitors (CPI), therapeutic vaccines significantly enhance anti-tumor and clinical responses. PharmaJet needle-free delivery can improve DNA vaccine delivery and has been successfully adopted into multiple vaccine development programs while demonstrating robust immunogenicity, with favorable clinical outcomes for novel therapeutic vaccines.
The poster presentation will highlight recent partner study results showing immunogenicity improvement when delivered with the PharmaJet Stratis® including:
- Evaxion: In Phase 1/2 trials the Stratis Needle-free Delivery System was used to administer EVX-02 DNA vaccine in combination with CPI (nivolumab) to patients that had a complete resection of Stage IIIB/IIIC/IIID or Stage IV melanoma and were a high risk of recurrence. The study, comparing Stratis delivery to poloxamer delivery with needle/syringe, showed EVX-02 was immunogenic with an improvement in T cell induction when using Stratis compared to needle/syringe. These patients were relapse-free at their last assessment.2 The 2nd generation EVX-03 vaccine induced a more potent response in a pre-clinical model.
- Scancell: The Phase 2 trial with patients that have advanced unresectable melanoma who have received the SCIB1 or ISCIB1+ DNA vaccine with CPI, either with nivolumab and ipilimumab or pembrolizumab is ongoing. Latest data shows SCIB1 administered with Stratis has induced T cell responses in 87% of patients who have been given double CPI and showed a significant increase from baseline T cell counts with full cohort data still to be analyzed.3 According to Lindy Durrant, Scancell CEO, “To date, Stratis is the only technology which has shown effective uptake of the DNA vaccine through intramuscular delivery allowing native cellular machinery to express the target antigen and induce a potent anti-tumor response.”4
“PharmaJet Needle-free Systems enable DNA cancer vaccine delivery and can be incorporated into both conventional and novel therapeutic strategies to treat various types of cancer,” noted Nathalie Landry, Chief Scientific Officer, PharmaJet. “These study results show that needle-free delivery is safe and well tolerated, induces antigen-specific T cell responses, and leads to favorable clinical outcomes.”
For more information visit the PharmaJet booth #533 from November 8-10, 2024, at the SITC conference, or visit the website at https://pharmajet.com.
Refer to Instructions for Use to ensure safe injections and to review risks.
1 Ledesma-Feliciano C, et al (2023). Improved DNA Vaccine Delivery with Needle-free Injection Systems. Vaccines. 11(2):280
2 Keline-Kohlbrecher et al (2023). Ai-designed personalized neoantigen vaccine, EVX-02, Induces robust T-cell responses in melanoma patients. Society for Immunotherapy of Cancer (SITC) 2023 poster.
3 Paston, et al (2024). A DNA plasmid melanoma cancer vaccine, SCIB1, combined with nivolumab + ipilimumab in patients with advanced unresectable melanoma. Cancer immunotherapy as Association (CIMT) 2024 poster.
4 Lindy Durant, Professor and CEO Scancell, Scancell Holdings Chief Executive Officer’s Report, September 24, 2024.
About PharmaJet
The PharmaJet mission is to improve the performance and outcomes of injectables with our enabling technology that better activates the immune system. We are committed to helping our partners realize their research and commercialization goals while making an impact on public health. PharmaJet Precision Delivery Systems™ can improve vaccine effectiveness and promote a preferred patient and caregiver experience while being safe, fast, and easy-to-use. The Stratis® System has U.S. FDA 510(k) marketing clearance, CE Mark, and WHO PQS certification to deliver medications and vaccines either intramuscularly or subcutaneously. The Tropis® System has CE Mark and WHO PQS certification for intradermal injections. They are both commercially available for global immunization programs. For more information or if you are interested in partnering with PharmaJet to improve the impact of your novel development program, visit https://pharmajet.com or contact PharmaJet here. Follow us on LinkedIn.
ABOUT SITC
The Society for Immunotherapy of Cancer (SITC) is the world’s leading member society of medical professionals dedicated to advancing cancer immunotherapy and biological therapy through its initiatives, educational sessions, and collaborative endeavors. SITC has become the premier forum for innovative discussions in the field and remains a central provider of scientific information for its diverse members serving in academia, industry, and regulatory agencies in the U.S. and abroad.
Contacts
Nancy Lillie
Nancy.Lillie@pharmajet.com
1-888-900-4321 Option 3




