- RT-114 yielded a relative bioavailability of 111% compared to PG-102 delivered subcutaneously with comparable PK profiles, meeting the primary endpoint of demonstrating bioequivalence –
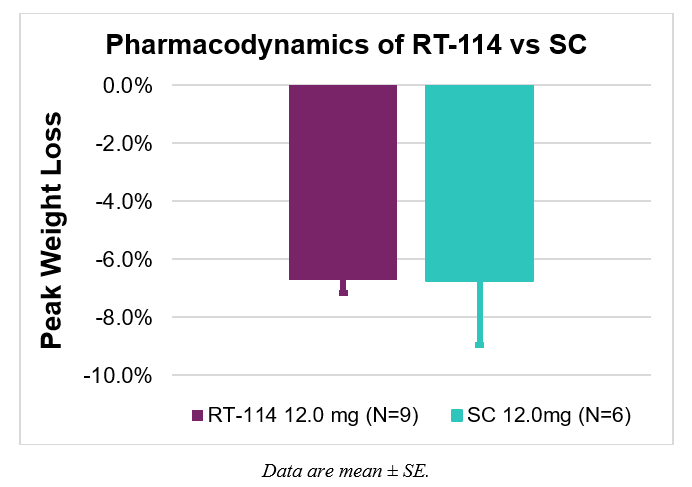
- Both groups demonstrated comparable weight loss with less variability observed with RT-114 compared to subcutaneous PG-102 –
- Phase 1 clinical trial of subcutaneous PG-102 demonstrated weight loss in obese patients, with an average reduction of 4.8% and up to 8.7% following five weeks of dosing -
- Data bolster expanding body of evidence of the RaniPill® platform’s potential to facilitate oral delivery of multiple obesity treatments including previously announced semaglutide -
- Phase 1 clinical study for RT-114 for the treatment of obesity expected to initiate in mid- 2025 -
SAN JOSE, Calif., March 26, 2025 (GLOBE NEWSWIRE) -- Rani Therapeutics Holdings, Inc. (“Rani Therapeutics” or “Rani”) (Nasdaq: RANI), a clinical-stage biotherapeutics company focused on the oral delivery of biologics and drugs, today announced pharmacokinetic and pharmacodynamic data from a preclinical study evaluating RT-114, a GLP-1/GLP-2 dual agonist (PG-102). PG-102 delivered orally via the RaniPill® capsule demonstrated comparable bioavailability and weight loss to subcutaneously (SC) injected PG-102 (“SC PG-102”). PG-102 is ProGen Co., Ltd’s (“ProGen”) Fc-fusion protein conjugated GLP-1/GLP-2 dual agonist.
“We believe that RT-114 has the potential to be a first-in-class, orally administered GLP-1/GLP-2 dual agonist for the treatment of obesity, addressing a critical gap in the treatment landscape. Despite the remarkable success of GLP-1 receptor agonists, there is a pressing need for effective oral therapies with convenient dosing strategies to eliminate the need for burdensome injections. With RT-114’s extended half-life, we are targeting a convenient, oral dosing regimen for the treatment of obesity,” said Talat Imran, Chief Executive Officer of Rani Therapeutics. “Furthermore, in our preclinical study, RT-114 achieved pharmacokinetics, bioavailability, and weight loss comparable to PG-102 delivered via subcutaneous injection. We believe that PG-102’s GLP-1/GLP-2 dual agonist construct is key to its potential to induce higher quality weight loss, positioning RT-114 to potentially deliver improved body composition and nutritional health as an oral therapy. We are encouraged by the data generated to date and look forward to advancing RT-114 into a Phase 1 clinical trial this year.”
RT-114 represents the fourth incretin-based drug to be studied preclinically in the RaniPill® capsule or via the RaniPill® route of delivery. In February 2025, Rani announced preclinical data of semaglutide delivered via the RaniPill® capsule.
ProGen recently announced preliminary results of the repeat-dose portion (Phase 1C) of its Phase 1 study where SC PG-102 demonstrated weight loss in obese subjects, with an average reduction of 4.8% and up to 8.7% following five weeks of dosing (30/60/80/80/80mg). SC PG-102 demonstrated tolerability, while reaching the target dose within one month. Among 73 patients who received SC PG-102 across the entire Phase 1 program, there were no treatment discontinuations. In previously disclosed preclinical data, PG-102 demonstrated improved body composition (fat vs. lean mass loss) compared to tirzepatide and dapiglutide in a diet-induced obese mouse model. Additional data on PG-102 will be presented at the upcoming Asian Association for the Study of Diabetes (AASD) conference this week.
“First with semaglutide and now with RT-114, Rani has demonstrated comparable pharmacokinetics and weight loss outcomes in an oral delivery at the same dose as their respective injectable counterparts. This achievement is unprecedented when compared to existing oral GLP therapies on the market and in development,” said Jesper Høiland, Senior Strategic Advisor at Rani Therapeutics and former President & EVP, USA at Novo Nordisk. “Furthermore, the short titration schedule and promising tolerability profile observed with subcutaneous PG-102 in the Phase 1C study may facilitate a quicker onset of effect. This could address a significant challenge with current GLP-1 treatment options, where patients typically do not experience clinically meaningful weight loss until after 4 to 5 months of treatment. By combining these potential advantages of PG-102 with the convenience of an oral delivery, Rani has the opportunity to create a truly differentiated product profile with RT-114.”
RT-114 Study Design
- The preclinical study was a head-to-head comparison of orally administered PG-102 via RaniPill® capsule (RT-114) and subcutaneously administered PG-102 in 16 healthy canines.
- All canines received a 12mg dose of PG-102 either via RaniPill® capsule (RT-114) (N=10) or subcutaneously (N=6), which is estimated to be approximately equivalent to a 60mg dose in humans.
- Endpoints included measures of safety, tolerability, and reliability of the RaniPill® capsule, as well as pharmacokinetics (serum drug concentration determined by ELISA) and pharmacodynamics (body weight and food intake).
Data Highlights
- The RaniPill® capsule was well tolerated with no changes in drug-related safety profile compared to subcutaneous delivery and was excreted without sequelae in all canines.
- RT-114 achieved a 90% delivery success rate (9 out of 10 canines).
- RT-114 yielded higher Cmax, earlier Tmax, and a relative bioavailability of 111% compared to SC PG-102.
- Average peak weight loss was the same in both groups with greater variability with SC dosing (6.7% ± 0.5% for RT-114 and 6.7% ± 2.2% for SC PG-102).
- Rises in serum drug levels coincided with decreases in food consumption indicating strong PK-PD relationship.
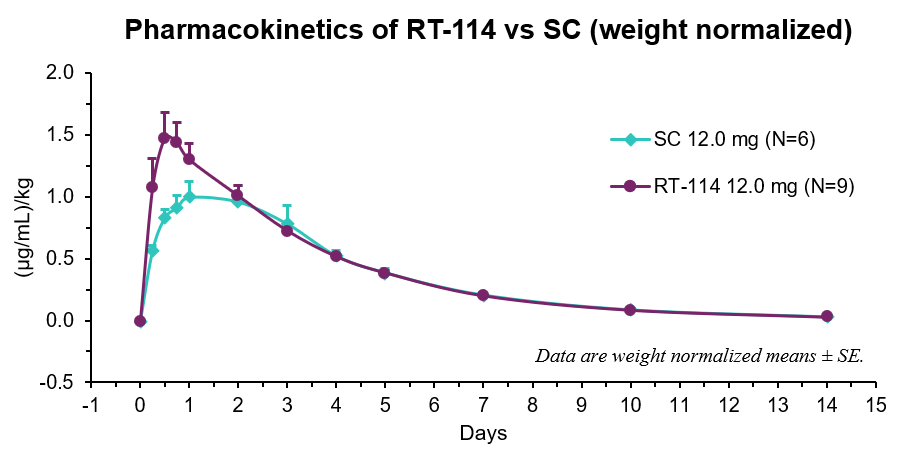
| Route | Cmax (µg/mL)/kg | Tmax (days) | AUClast (µg/mL*day)/kg |
| RT-114 | 1.51 ± 0.19 | 0.7 ± 0.1 | 5.48 ± 0.33 |
| SC | 1.06 ± 0.13 | 1.3 ± 0.3 | 4.92 ± 0.52 |
Near-Term Milestone Expectations:
- Initiation of Phase 1 clinical trial of RT-114 for the treatment of obesity expected in mid-2025.
About Rani Therapeutics
Rani Therapeutics is a clinical-stage biotherapeutics company focused on advancing technologies to enable the development of orally administered biologics and drugs. Rani has developed the RaniPill® capsule, which is a novel, proprietary and patented platform technology, intended to replace subcutaneous injection or intravenous infusion of biologics and drugs with oral dosing. Rani has successfully conducted several preclinical and clinical studies to evaluate safety, tolerability and bioavailability using RaniPill® capsule technology. For more information, visit ranitherapeutics.com.
About RT-114 Collaboration
RT-114 is the subject of a Collaboration Agreement between Rani and ProGen entered into in June 2024. Under the Collaboration Agreement, Rani and ProGen will collaborate to manufacture, develop, seek regulatory approvals for and, if approved, commercialize RT-114 in the field of weight management (including without limitation obesity, weight reduction and weight maintenance) in humans. Under the Collaboration Agreement, development costs, as well as operating profits and losses from the commercialization of RT-114, will be equally shared by Rani and ProGen. The parties share responsibility for the development of RT-114 worldwide, with Rani leading such development for preclinical activities through Phase 1 clinical trials. After initiation of the first Phase 2 clinical trial, Rani will lead development and commercialization of RT-114 in the United States, Canada, Europe (including the United Kingdom) and Australia, and ProGen will lead development and commercialization in all other countries.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements include statements regarding, among other things, the expected initiation of a Phase 1 trial of RT-114 in mid-2025, the potential of the RaniPill® platform to enable oral delivery of multiple obesity treatments and validation of such potential through preclinical data, the potential for RT-114 to be a first-in-class orally administered GLP-1/GLP-2 dual agonist to treat obesity, the targeting of RT-114 to have a convenient oral dosing regimen for the treatment of obesity, the potential of PG-102’s GLP-1/GLP-2 dual agonist construct to induce higher quality weight loss, the potential for RT-114 to improve body composition and nutritional health of patients, the convenience and attractiveness of an oral RT-114 treatment for patients, the potential that the characteristics of PG-102 could lead to faster onset of effect and achievement of clinically meaningful weight loss, the sufficiency of Rani’s cash reserves, and future financial performance. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “believe,” “would,” “potential,” “expect,” “targeting,” “may,” “could,” “look forward” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon Rani’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks and uncertainties associated with Rani’s business in general and the other risks described in Rani’s filings with the Securities and Exchange Commission, including Rani’s annual report on Form 10-K for the year ended December 31, 2023, and subsequent filings and reports by Rani. All forward-looking statements contained in this press release speak only as of the date on which they were made and are based on management’s assumptions and estimates as of such date. Rani undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
Investor Contact:
investors@ranitherapeutics.com
Media Contact:
media@ranitherapeutics.com
Graphs accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/a05200b3-2a45-4fde-b650-a37e283a2843
https://www.globenewswire.com/NewsRoom/AttachmentNg/29308181-ece1-4852-9667-287d84f843c6





