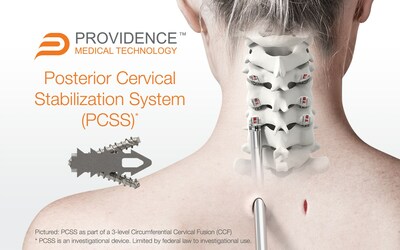
Providence Medical Technology, Inc. (PMT) today announced the close of enrollment in its FUSE Study — a prospective, multicenter, randomized, Investigational Device Exemption (IDE) clinical study evaluating the safety and effectiveness of its Posterior Cervical Stabilization System (PCSS) in 3-level cervical fusion patients.
|
PLEASANTON, Calif., May 11, 2023 /PRNewswire/ -- Providence Medical Technology, Inc. (PMT), a leader in medical device innovation for spine surgery, today announced the close of enrollment in its FUSE Study — a prospective, multicenter, randomized, Investigational Device Exemption (IDE) clinical study evaluating the safety and effectiveness of its Posterior Cervical Stabilization System (PCSS) in 3-level cervical fusion patients. The study enrolled approximately 230 patients across 18 leading spinal surgery sites in the United States to evaluate PCSS in combination with Anterior Cervical Discectomy and Fusion (ACDF) compared to ACDF alone in a superiority study. The study began in May 2020 and is designed to evaluate Circumferential Cervical Fusion (CCF) in high-risk cervical fusion patients by producing level-1 clinical evidence. The study includes patients at risk for failure of a standard ACDF fusion procedure due to risk factors such as 3-level disease, smoking, advanced age, and diabetes. While a traditional ACDF procedure is generally associated with positive outcomes in otherwise healthy patients, the success rate for these high-risk patients is significantly less favorable. Of the over 300,000 anterior cervical fusion procedures performed annually, roughly 30% involve patients with significant risk factors for nonunion. This study is investigating whether these patients could benefit from a tissue-sparing posterior cervical fusion as an adjunct to the ACDF. The first comparative analysis for fusion and other clinical outcomes will occur when 100 patients reach two years of follow-up. The 100th patient is scheduled to return for their two-year follow-up visit at the end of 2023. "The completion of FUSE enrollment is an important milestone for high-risk spinal fusion patients," said Jeff Smith, CEO and Founder of Providence. "At Providence, we are on a mission to establish CCF as the standard of care for high-risk patients. We believe these patients require more and are hopeful the FUSE results will raise awareness of the extensive unmet need in the treatment of patients with risk factors for nonunion. These are normal patients seen by surgeons routinely, however, are often excluded from spine fusion clinical studies because it is well-understood that they have worse outcomes. The FUSE Study is the largest ever level-1 study on high-risk cervical fusion patients, and I am proud of our multi-year commitment and our amazing progress to date. We are grateful to our dedicated team of surgeons, research coordinators, nurses, contractors, and support staff who worked tirelessly on this mission. We look forward to performing our initial analysis of this superiority study." Half of the patients in the FUSE Study received anterior fusion surgery involving a plate and screws, a common surgery and the current standard of care. The other half received an anterior fusion plus a posterior fusion using the company's CORUS™ Spinal System and PCSS titanium implants. The CORUS Spinal System is a unique set of instruments used to perform a tissue-sparing posterior cervical fusion. The Posterior Cervical Stabilization System (PCSS) is an investigational device consisting of non-segmental instrumentation with integrated screw fixation intended to provide immobilization and stabilization of spinal segments. PCSS achieves bilateral facet fixation by spanning the interspace at each level with points of fixation at each end of the construct. Some of the leading FUSE study investigators also added comments on their experience in the trial using the CORUS Spinal System and the investigational PCSS device: "I have enjoyed being a part of the FUSE study. I have been impressed with my personal experience using the CORUS Spinal System and am looking forward to assessing the impact of the PCSS implants on clinical outcomes at one and two years. Being a part of this study has caused me to re-think how I treat patients requiring cervical fusions who are at a somewhat higher risk of not fusing. In my practice going forward, those higher-risk patients will very likely be getting a posterior cervical fusion using the CORUS Spinal System as an adjunct to ACDF." – K. Brandon Strenge, MD, The Orthopaedic Institute of Western Kentucky (FUSE Study Investigator). "Congratulations to Providence, the investigators, and most importantly, the patients for reaching this amazing milestone. This has been an exciting study to participate as a principal investigator. I am eager to start analyzing the data and reporting the overall outcomes. This study has the potential to produce impactful discoveries in the coming months and years." – Pierce D Nunley, MD, Director, Spine Institute of Louisiana (FUSE Study Investigator). "I have used the CORUS Spinal System for less invasive posterior cervical fusion for some time, and it has been the most impactful change in my practice, for the better. As part of the FUSE study, I look forward to evaluating the effect CORUS may have in combination with PCSS implants for 3-level high-risk patients." – Alex Lemons, MD Orthopaedic Surgery, Pinehurst Surgical Clinic (FUSE Study Investigator). "This study is designed to ultimately determine the value of 360 fusion in high-risk patients and the use of minimally invasive techniques to deliver better outcomes for our patients. I believe this technique offers less morbidity than lateral mass screw fixation. This could provide benefit to this underserved, high-risk patient population." – Jamieson Glenn, MD, Orthopaedic Spine Surgeon, Scripps, Encinitas (FUSE Study Investigator). CAUTION: The PCSS is an investigational device. Limited by Federal (United States) law to investigational use. About Providence Medical Technology Providence Medical Technology, Inc. is a privately held medical device company focused on improving clinical outcomes and preventing failures for high-risk spinal surgery patients. The company has pioneered a proprietary approach to posterior cervical fusion and has developed surgical instrumentation and implants that offer unique benefits to the more traditional surgical approaches and implants. Providence Medical Technology products have been used in over 18,000 patients worldwide. The Providence family of products includes the CORUS™ Spinal System, CAVUX® cages, ALLY® bone and facet screws, and ENTRUS™ Allograft Bone. All implants and instruments are sterile-packaged and single-use to maximize perioperative efficiency and ensure consistent quality and performance. For more information, please visit providencemt.com/pcss and fusestudy.com.
SOURCE Providence Medical Technology, Inc. |