On October 22, Qilu Pharmaceutical unveiled the latest findings from a multicenter, single-arm Phase II clinical trial at the European Society for Medical Oncology (ESMO) Annual Congress 2023.
| |
| [25-October-2023] |
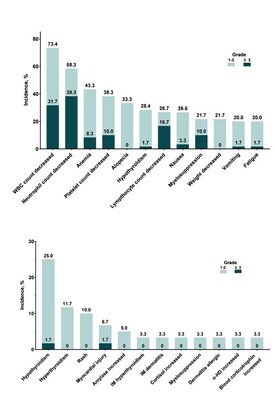
| JINAN, China, Oct. 25, 2023 /PRNewswire/ -- On October 22, Qilu Pharmaceutical unveiled the latest findings from a multicenter, single-arm Phase II clinical trial at the European Society for Medical Oncology (ESMO) Annual Congress 2023. This trial studied the use of QL1706 (iparomlimab and tuvonralimab) in combination with chemotherapy, with or without bevacizumab, as a first-line treatment of recurrent or metastatic cervical cancer (r/mCC). Professor Danbo Wang from Liaoning Cancer Hospital & Institute reported these findings. The study results indicated that using QL1706 in combination with chemotherapy, with or without bevacizumab, as a first-line treatment for r/mCC showed a promising objective response rate (ORR) and survival benefits. Moreover, the treatment exhibited a manageable safety profile without any new safety signals observed, making it a potential new first-line treatment option for r/mCC patients. I. Research Background Currently, the preferred standard first-line treatment for r/mCC patients is cisplatin or carboplatin and paclitaxel plus bevacizumab, and efficacy and safety of the treatment also need to be considered. For r/mCC patients with positive PD-L1 expression, the PD-1 inhibitor pembrolizumab, administered with chemotherapy and with or without bevacizumab, is recommended as the standard first-line treatment. Immunotherapy combined with chemotherapy has now become the standard care for r/mCC [1-2]. The latest results from the KEYNOTE-826 study showed that the median progression-free survival (PFS) of PD-L1-positive (CPS ≥1) r/mCC treated with immunotherapy-chemotherapy-pembrolizumab, with or without bevacizumab, increased from 8.2 months to 10.4 months. Furthermore, the median overall survival (OS) extended from 16.5 months to 28.6 months. While the PD-1 inhibitor combined with chemotherapy have significantly improved patient survival compared to chemotherapy alone, the survival benefits for patients remain limited, indicating an unmet clinical need [3-4]. II. Study Design This study enrolled r/mCC patients who had not undergone systemic therapy. These patients were treated with either QL1706-chemotherapy (Cohort 1) or QL1706-chemotherapy-bevacizumab (Cohort 2) until disease progression, the occurrence of intolerable toxicity or the patient’s withdrawal of informed consent. The primary endpoint of the study was safety. Secondary endpoints included ORR, duration of response (DOR), disease control rate (DCR), PFS, which were assessed by the investigators according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) and OS. The study design is illustrated in Figure 1. III. Study Results A total of 60 patients were enrolled to cohort 1 and cohort 2, 30 patients in each cohort. Patients in cohort 1 were treated with QL1706 monoclonal antibody combined with either cisplatin or carboplatin and paclitaxel. Patients in Cohort 2 received the same regimen as the first but with addition of bevacizumab. The mean age of the patients was 52.0 years, with 58.3% having an ECOG Performance Status of 1. Squamous cell carcinoma was the pathological type in 78.3% of the patients, while recurrent CC was found in 86.7%. As of April 24, 2023, the median follow-up was 14 months. Out of 58 patients who received at least one post-baseline efficacy evaluation, ORR was 81.0% (95% CI, 68.6-90.1). Among these patients, eight achieved complete response (CR) and 39 achieved partial response (PR). The DCR was 98.3% (95% CI, 90.8-100.0). The median PFS reached 14.3 months (95% CI, 9.2 months to not estimable), while the median OS had not yet been reached. In cohort 2, with combination treatment of bevacizumab, the median PFS was 16.4 months. (Figure 2) In terms of safety, treatment-related adverse events (TRAEs) were observed in all patients, with a 71.7% incidence of grade 3 or higher adverse events. The most common TRAEs included white blood cell count decreased (71.3%), neutrophil count decreased (68.3%), and anemia (43.3%). The incidence of treatment-related serious adverse events was 30%; the occurrence of immune-related adverse events (irAEs) was 13.3%; TRAEs leading to discontinuation of therapy occurred in 26.7% of patients; and the incidence of treatment-related deaths was 1.7%, possibly related to bevacizumab (Figure 3). IV. Summary The results of this study demonstrated that the first-line treatment of r/mCC with QL1706 combined with chemotherapy, with or without bevacizumab, showed promising efficacy and favorable safety, regardless of level of PD-L1 expression. Based on these findings, a Phase III clinical trial of QL1706 in combination with chemotherapy, with or without bevacizumab, for the treatment of persistent, recurrent, or metastatic CC is currently ongoing. Furthermore, the New Drug Application (NDA) for the treatment of r/mCC patients who have failed at least one first-line standard platinum-based therapy with QL1706 was accepted by the Center for Drug Evaluation (CDE) in August of this year. References: 1. Guidelines Working Committee of the Chinese Society of Clinical Oncology. Guidelines for the Diagnosis and Treatment of Cervical Cancer [M]. 2023. Beijing: People’s Medical Publishing House, 2023:70. 2. Tewari KS, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014 Feb 20;370(8):734-43. doi: 10.1056/NEJMoa1309748. Erratum in: N Engl J Med. 2017 17;377(7):702. 3. Colombo N, et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N Engl J Med. 2021 11;385(20):1856-1867. 4. J Clin Oncol 41, 2023 (suppl 16; abstr 5500).
SOURCE Qilu Pharmaceutical Co., Ltd. |