Qvin™, the biotechnology research company that developed the first and only healthcare service that collects menstrual blood samples as an alternative to traditionally collected venous blood draws, announced FDA clearance of its Q-Pad™ and A1c Test.
Qvin Receives its First FDA Clearance for Q-Pad and Q-Pad A1c Test
SAN FRANCISCO, Jan. 8, 2024 /PRNewswire/ -- Qvin™, the biotechnology research company that developed the first and only healthcare service that collects menstrual blood samples as an alternative to traditionally collected venous blood draws, today announced FDA clearance of its Q-Pad™ and A1c Test. The clearance makes it possible for the millions of women in America who live with diabetes to receive monitoring of A1c, using laboratory tests performed on the Q-Pad. More broadly, this marks an opportunity for testing important biomarkers for the more than 80 million people who menstruate in the U.S.
The traditional methods of blood testing require invasive procedures administered by medical professionals, and those can be time-consuming and expensive. Not everyone has the time, access, and financial means to get laboratory results for blood work, however, billions of people globally have their period every single month. And yet, menstrual samples had never previously been explored as a diagnostic source for health information. Qvin proved the clinical relevancy of menstrual blood for a number of important biomarkers. Now, for the first time, menstrual blood can be used to provide insights for people with the Q-Pad and A1c Test, from the convenience of their own home. FDA clearance of the Q-Pad and A1c Test, for at-home collection of samples and over-the-counter (OTC) distribution, speaks to the safety and simplicity of the Q-Pad technology.
"With the first ever FDA-cleared menstrual blood health test, Qvin is paving the way to important new opportunities for women's health and this is just the beginning," said Dr. Sara Naseri, Qvin Co-founder, Medical Doctor, and scientist. "We are simplifying routine testing, and freeing up resources that can be used on providing care and ultimately our goal is to make health care much more accessible."
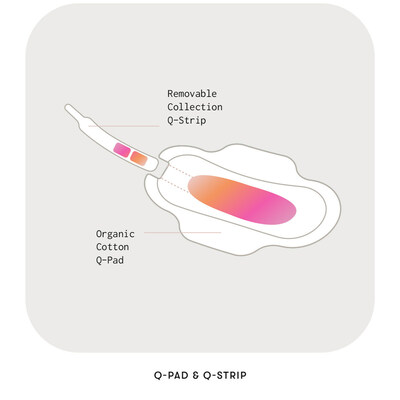
Each Q-Pad includes a removable strip; once the Q-Pad has sufficiently collected a menstrual sample, the removed collection strip is sent to a CLIA-Certified (Clinical Laboratory Improvement Amendments) laboratory for clinical testing. Users receive their results via the free and convenient Qvin app.
The Qvin A1c Q-Pad Test Kit measures the average blood sugar over a three-month period, by testing the A1c biomarker for people with diabetes. It's one of the most commonly used tests to monitor diabetes and pre-diabetes. Blood sugar (glucose levels) can have health impacts, even for non-diabetics. This test allows patients to track their blood sugar.
"Utilizing menstrual samples, the Q-Pad can address critical women's health issues that have historically been neglected," stated Søren Therkelsen, Co-founder of Qvin. "Because of our vertically integrated infrastructure, we will over time be able to deliver healthcare solutions at a significantly lower cost than traditional methods. We are proud to have developed a product that has the potential to vastly improve access to global healthcare."
Qvin, in collaboration with researchers at academic institutions such as Stanford University School of Medicine, has published peer-reviewed research validating other biomarkers that can also be monitored. Q-Pad allows individuals to submit specimens directly to the lab and receive reports on key health conditions that often go undiagnosed or misdiagnosed including pre/diabetes, anemia, fertility, perimenopause, endometriosis, and thyroid health.
"The research and development that Qvin has undertaken is both highly novel and innovative in helping women better address their health concerns," said Dr. Paul Blumenthal, Professor Emeritus of Obstetrics and Gynecology at Stanford University, and an author on multiple Qvin publications. "For instance, women seeking to understand their fertility status can soon monitor various reproductive hormones via menstrual blood using the Q-Pad. In addition, published research indicates that the Q-Pad could be a convenient, user-friendly, and efficient way of screening for the Human Papilloma Virus (HPV) as part of global cervical cancer prevention efforts."
Learn more about Q-Pad™ Kit availability and price on Qvin.com
About Qvin™
Qvin™, whose name stems from the Danish word for women, is on a mission to empower people who menstruate by giving them access to regular insights about their health for early detection and monitoring. The Q-Pad is non-invasive, convenient, and can be used for testing anywhere in the world. Qvin eliminates common barriers to traditional laboratory testing, which can be difficult for many globally due to lack of access, time, or financial means. Removing these barriers to care can lead to better and more accurate diagnoses and health management. The Q-Pad was initially created to identify biomarkers for HPV and has expanded and identified additional biomarkers to test for, including pre/diabetes, anemia, fertility, perimenopause, endometriosis, and thyroid health. To learn more, visit https://www.qvin.com.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/qvin-introduces-q-pad-transforming-womens-health-with-fda-cleared-lab-testing-using-menstrual-blood-302027835.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/qvin-introduces-q-pad-transforming-womens-health-with-fda-cleared-lab-testing-using-menstrual-blood-302027835.html
SOURCE Qvin